Ionic Bond is also known as Electrovalent Bond. It’s a kind of linkage composed with the help of electrostatic attraction in between charged ions that are opposite in nature, relating to a chemical compound.
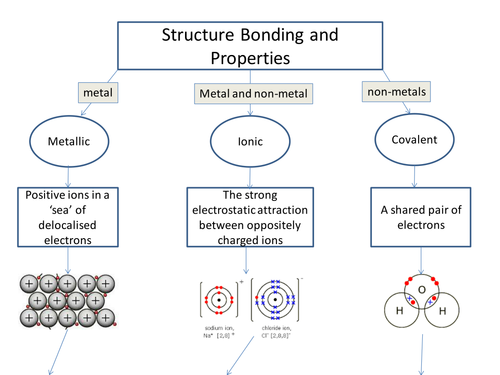
Such bond is formed when outer shell electrons or valence electrons of a single atom are permanently transferred to the other atom. The atom which is losing electrons turns into a “Cation”, which is known to the “positively charged ion”, on the other hand, the one that gains electrons are the negatively charged ion, known as the Anion.
Let’s take an example of bonding in Sodium Chloride (NaCl)
- Sodium or Na consists of configuration (2,8,1). In this configuration, one electron in the outermost shell i.e. (in M shell out of K, L, M, and N shell) extra and by donating that one electron, it will become more stable resulting in noble gas configuration (2,8).
- And in Chlorine (Cl) having the configuration (2,8,7) is lacking with one electron to achieve the stable noble gas configuration of (2,8,8). If it’s able to gain one extra electron from anyone, then it will become stable.
So, on a whole, if Na atom gives an electron to Cl atom, both will achieve stability. This diagram will further illustrate it:
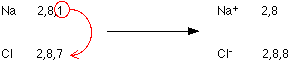
In the above diagram, the Na atom loses an electron and accordingly it does not consist of equal proton and electron numbers. The reason is – it has 1 extra proton than an electron and it acquires a positive charge (1+).
Whereas Chlorine has accepted the electron, accordingly now, it consists of 1 extra electron than a proton. It thus includes a negative charge (1-).
The bonding in Ionic makes compounds called Ionic compounds or Electrovalent compounds, that are rightly demonstrated by the formation of compounds between alkali, alkaline-earth metals, and non-metals.
In case of Ionic bonds in crystalline solids, the electrostatic force of attraction or the Coulomb Force, or Coulomb Attraction held between repulsion and opposite charges between identical charges position the ions in a way that each and every cation (positive ion) gets surrounded by anion (negative ion) and every negative ion is enclosed by positive ion.
Briefly, the ions are arranged in a manner so that positive and negative charge alternate and also balance each other, so that the overall charge of the whole substance is counted as zero.
Electrostatic forces’ magnitude in the ionic crystal is large, considerable, and in abundance. Accordingly, these substances are likely to be tough, hard, compacted, and non-volatile.
The Ionic Bond is indeed an extreme condition of “Polar Covalent Bonds”, the latter arising from uneven distribution of electrons instead entire electron transfer. Ionic bonds are usually formed in between the two atoms whose electronegativity difference is very large, while a molecular or covalent bond is formed when electronegativities are identical. Finally, have a comparison on the covalent bond.
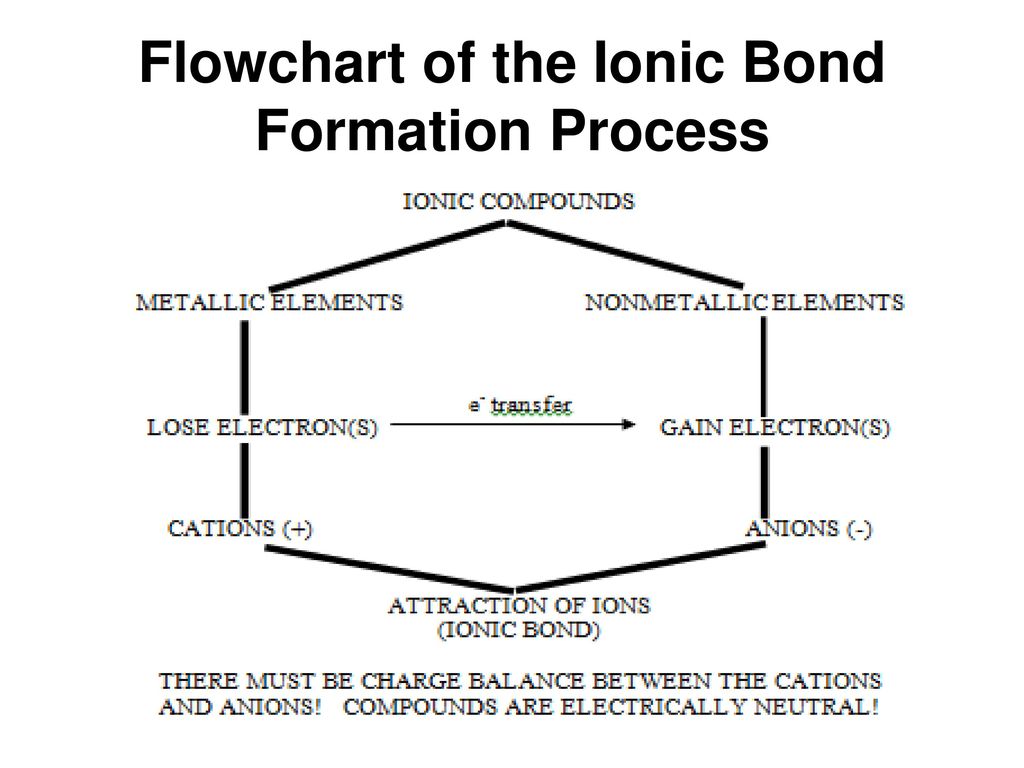
There is a complete transfer of the valence electrons in Ionic Bonding in between the atoms. Also, it’s a kind of chemical bond, which produces two opposite charged ions. This is noticed due to the metals with some electrons in their outermost orbital.
By the loss of those electrons, these metals have the ability to obtain the configuration of noble-gas and meet the Octet Rule. Likewise, non-metals which have electrons close to eight in its outermost/valence shell contributes to quickly accept electrons in order to attain the Noble Gas Configuration.
The concluded overall energy involved in the process of ionic bonding that includes ionization energy of metal and nonmetal’s electron affinity, which is generally positive. This indicates that the reaction is unfavorable and endothermic.