Chemical bonding is an integral part of any chemical reaction between two different elements. Atoms combine to attain stability. Formation of bonds is certainly important for the combination to take place. There are several different types of bonds that include electrovalent or ionic bonds, coordinate bonds and covalent bonds. Each bond is known to have several specific features associated to it.
A chemical bond is physically responsible for attractive interactions among atoms and molecules of an element. They also offer stability to diatomic and polyatomic chemical compounds. While there are various characteristics that could be realized regarding a chemical reaction using bond parameters. Knowing those could be of great importance. The major bond parameters have been discussed down below:
Bond Length
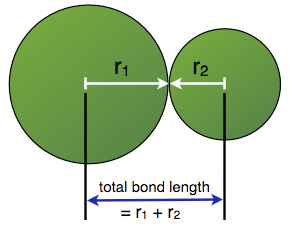
Also known as bond distance, bond length is defined as the distance between the nuclei of two different atoms that are bonded and positioned at equilibrium. Bond length could be measured using spectroscopic, electron diffraction or X-ray diffraction techniques. Smaller the bond length, stronger is known to be the force between two bonding atoms. Depending on the size of the atom, the bond length may increase. Each atom that is the part of the bond has its share in the bond length. For covalent bonds, the contribution from every atom is known as the covalent radius of the atom.
Factors that affect bond length
There are generally two factors that may affect the bond length. These include:
- Bond multiplicity: bond multiplicity and bond length are known to be inversely proportional. With a decrease in the bond multiplicity, bond length tends to increase.
- Size of an atom: The bond length and size of an atom are said to be directly proportional. If the size of an atom increases, the bond length will also increase.
Bond angle
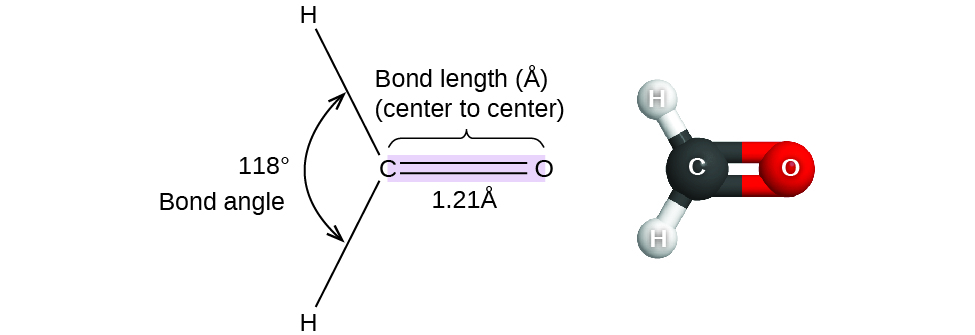
The angle formed by the orbitals containing the pairs of bonding electron around the central atom in the complex ion or molecule is called its bond angle. It is known to be measured in degrees and can be calculated through spectroscopic methods. The bond angle gives an idea about the distribution of electron pairs around the specific atom. Bond angle could be relevant for determining the shape of an atom.
Bond Enthalpy
Also referred as bond dissociation enthalpy, this is the strength of a chemical bond. It is the energy that would be required to break specific chemical bonds in one mole of gaseous molecule. Bond enthalpy is known to be directly proportional to the strength of bonds between the molecules. In case of polyatomic molecules, two bonds of the same type can possess different bond enthalpy. Due to prevalent differences between bond enthalpy, polyatomic bonds tend to have average bond enthalpy.
Bond order
According to the Lewis’ description of covalent bonds, Bond order is the number of bonds that could be formed between two atoms in a molecule. Isoelectric bonds or ions are said to possess the same bond order. With an increase in the bond order, bond enthalpy tends to increase and the bond length decreases.