Elements
An Element is defined as a material which cannot be changed or broken down into another substance using chemical means. It is a basic chemical building block of matter. It is made up of entirely from one type of atom. Atoms of the same element have the same atomic number or Z. For example, the hydrogen element is made from atoms containing a single proton and a single electron. If you change the number of protons in an atom, you will change the type of element it is.
In 1800, only 31 elements were known. At present 118 elements are known. Of them, the recently discovered elements are man-made. Efforts to synthesise new elements are continuing. With such a huge number of elements it becomes very difficult to study individually the chemistry of all these elements and their innumerable compounds individually. For this, scientists searched for a systematic way to organise their knowledge by classifying the elements. This would not only rationalize known chemical facts about elements, but even predict new ones for undertaking further study. Today elements are categorised using a periodic Table.
Periodic Table: The periodic table, is a tabular representation of the elements, which are arranged by atomic number, electron configuration, and recurring chemical properties.
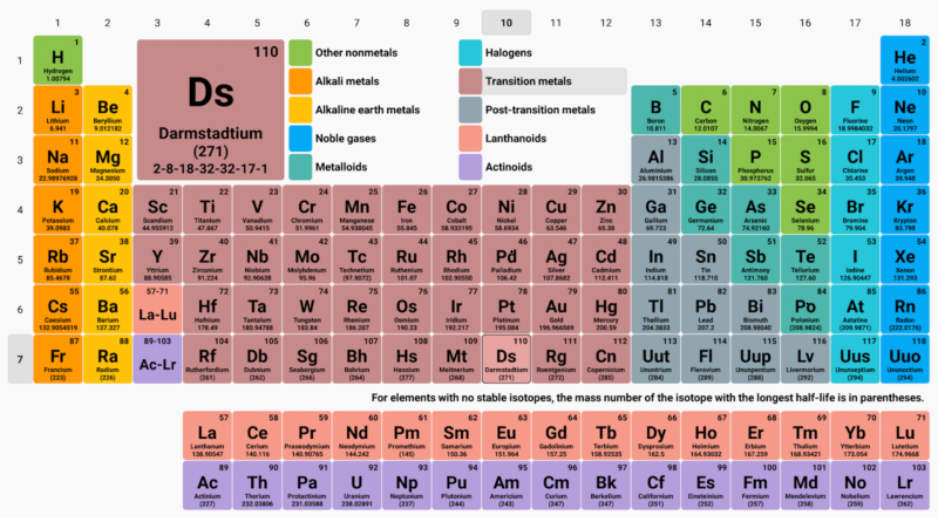
The structure of the table shows periodic trends. The seven rows of the table are called periods, and it generally has metals on the left and non-metals on the right. The columns are called groups; and it contains elements with similar chemical behaviours. The first period contains 2 elements and is the shortest period. Specific regions of the periodic table are referred to as blocks in recognition of the sequence in which the electron shells of the elements are filled. Each block is named according to the subshell in which the “last” electron resides.
The s-block comprises the first two groups which are alkali metals and alkaline earth metals as well as hydrogen and helium.
The p-block comprises the last six groups, which are groups 13 to 18 and contains, among other elements, all of the metalloids.
The d-block comprises groups 3 to 12 and contains all of the transition metals.
The f-block, has no group numbers and comprises lanthanides and actinides
Limitations of modern periodic table:
The modern periodic table suffers from several limitations. In the table, the position of hydrogen continues to remain ambiguous. The Inner transition elements are placed separately in the table as they do not find any place in the table. Nevertheless, the position of an element in the periodic table can be predicted easily. You can do this using electronic configuration of the element and the number of electrons in the valence shell.
Concept of Isotopes, Isobars and Isotones:
Isotopes: Atoms of the same element having different masses are called Isotopes i.e. different Atomic Mass (A) but same atomic number (Z) .
Isobars: Atoms of elements having same atomic mass but a different atomic number are called Isobars i.e. same A but different Z.