The atomic configuration can be defined by four quantum numbers (n, l, ml, ms) where ‘n’ is the principal quantum number, ‘l’ is the angular/orbital quantum number, ml is the magnetic quantum number and finally ‘ms’ is the electron spin quantum number. While ‘n’ denotes the number of electron shell i.e 1,2,3 and so on, ‘l’ denotes the shell and its shape i.e. s, p, d, f and so on. Within a subshell only (2l+1) orbitals a possible and the maximum occupancy of electrons is 2(2l+1). Hence, s subshell has 1 orbital that can occupy 2 electrons while p subshell has 3 orbitals that can occupy 6 electrons, d subshell has 5 orbitals that can occupy 10 electrons, f subshell has 7 orbitals with 14 electrons and so on (Huheey, Keiter, Keiter, & Medhi, 2006).
Likewise, ‘ml’ determines the number of orbitals and their orientation in the subshell and ‘ms’ specifies the direction of the electron spin i.e. +1/2, portrayed by ↑, or –1/2, portrayed by ↓. Therefore, two electrons may have ‘n’, ‘l’ and ‘ml’ value the same but the fourth quantum number must be different. Pauli’s exclusion principle corresponds with the electron spin and signifies the ability of an atom to generate a magnetic field.
Pauli’s exclusion principle states that in a poly electron system, no two electrons in an atom can have the same set of four quantum numbers. Therefore, only two electrons can exist in the same orbital and these electrons must have opposite spin. This means that if one electron is assigned up spin (+1/2), the other shall be assigned a down spin (-1/2) (Singh, 2011).
Wolfgang Pauli (1900-1958), an Austrian physicist was awarded a Nobel prize in 1945 for his exclusion principle. He made it clear that every electron in an atom occupies a discrete level of energy and spin. Therefore, when two electrons are placed in close vicinity, their outer energy level must shift slightly to allow discretion. As portrayed in Figure 1, a hydrogen atom has only one electron but the first three quantum numbers for helium are the same since both the electrons belong to the same energy level and same spherical shape. Therefore, the spin on these electrons differentiates them from one another.
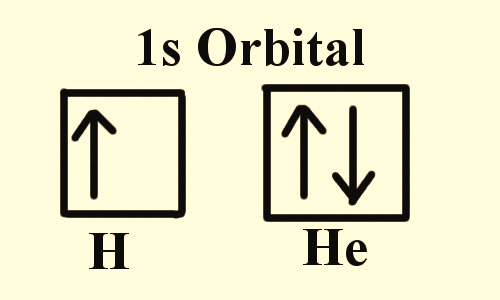
Figure 1: In an orbital filling diagram, the square represents an orbital and the arrows represent electrons of opposite spins. Electronic configuration of hydrogen and helium are presented in this figure.
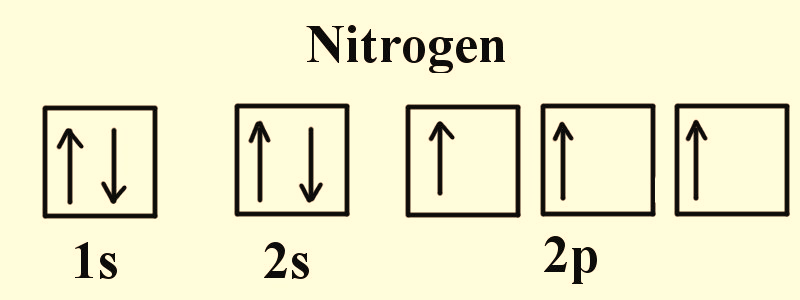
Figure 2: Electronic configuration of Nitrogen.
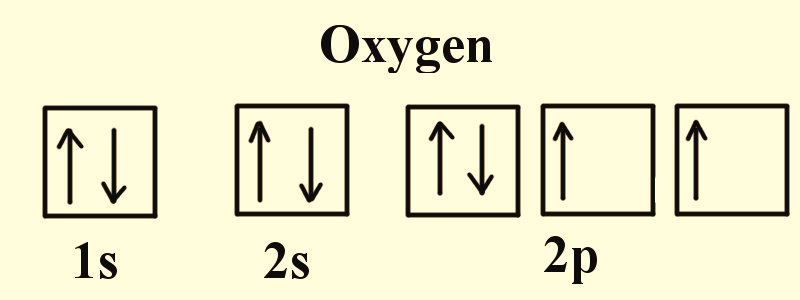
Figure 3: Electronic configuration of Oxygen.
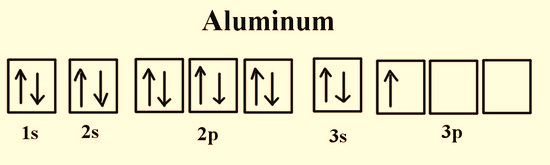
Figure 4: Electronic configuration of Aluminum.
All atoms except hydrogen have poly electron system. The physical and chemical properties of each element are directly correspondent to the position, shape, magnetic field and spin of the electrons that surround the neutral atom. Figure 2-4 indicate that in any element the electrons occupy the vacant orbitals singly and then the leftover electrons doubly occupy the orbitals. Since like charges repel each other, electrons prefer single occupancy in each orbital as the shielding effect of the nucleus is lesser on these electrons in comparison to double occupancy.