The atomic theory has evolved with the discovery of electrons, protons and neutrons and incorporated dual behavior of matter to propose a quantum mechanical model. An important section of this theory is the placement of electrons that surround the nucleus.
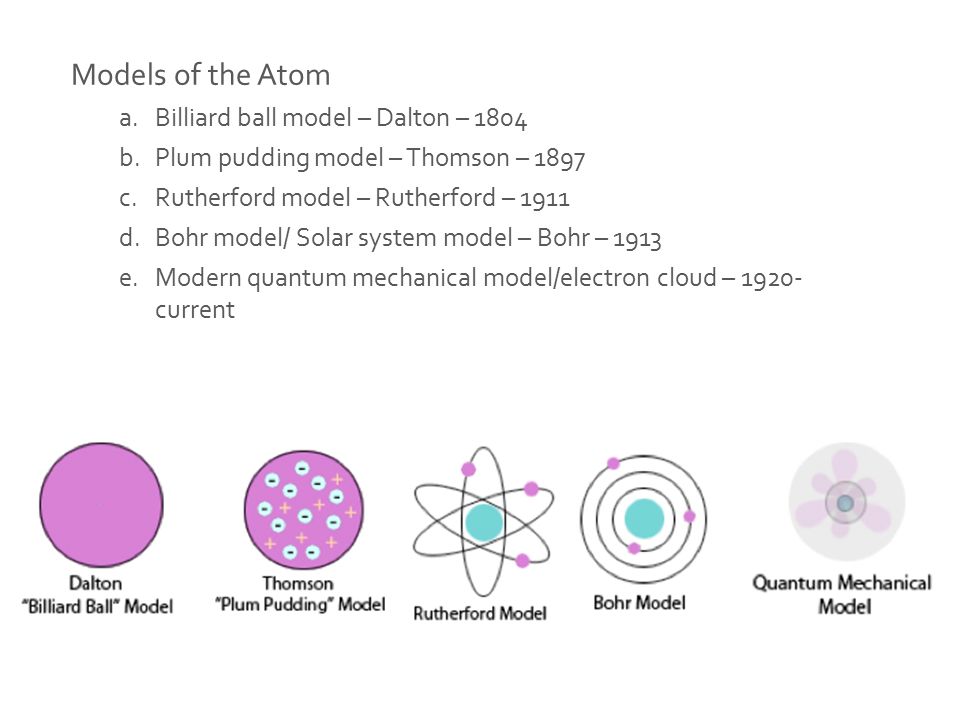
Figure 1: Evolution of atom from Billard ball model (1804), Plum pudding model (1897), Rutherford model (1911), Solar system model (1913) to modern Electron cloud model (1920-now)
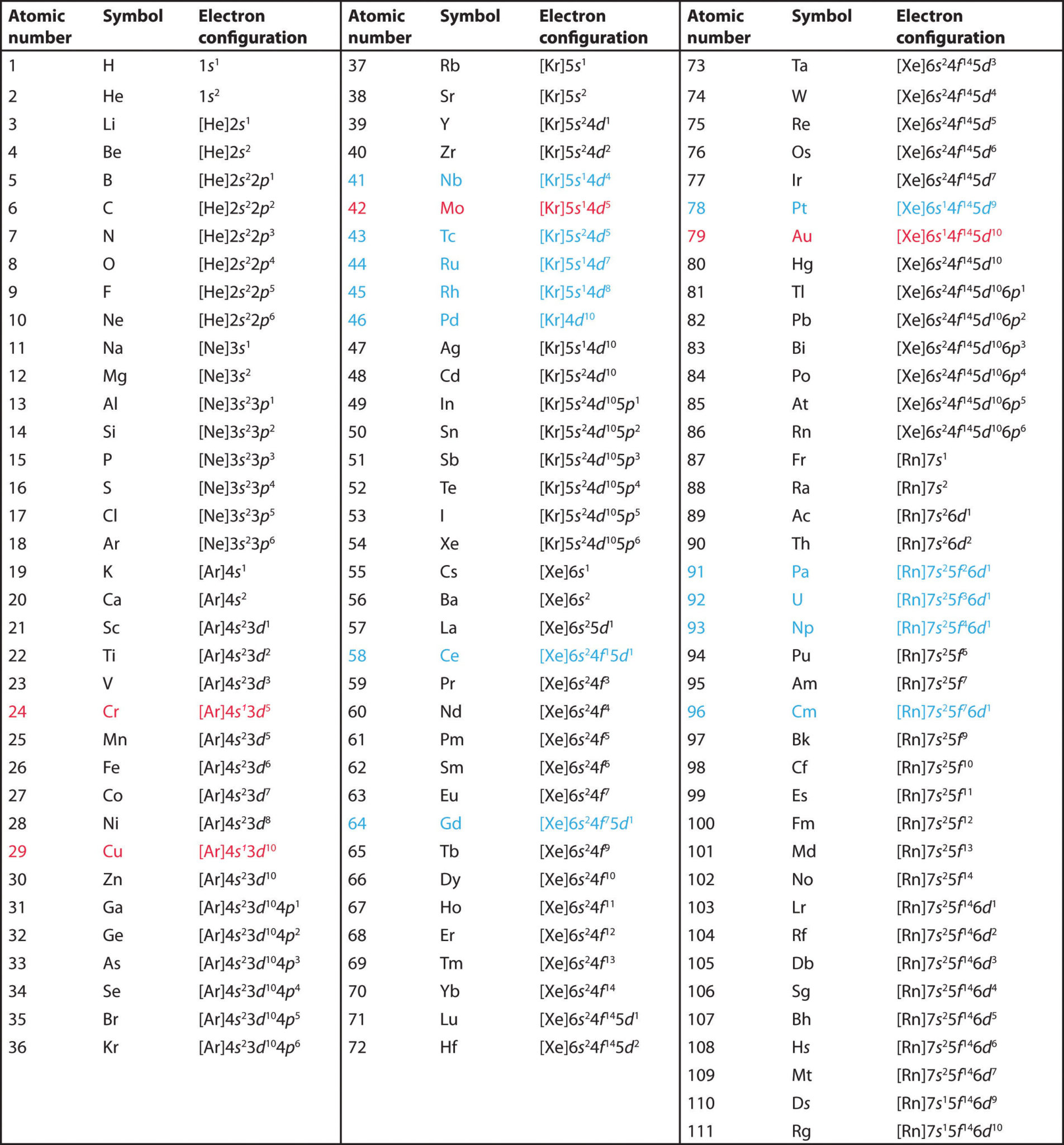
The distribution of electrons in different orbits was first suggested by Bohr and Bury. The electronic configuration helps us to understand the behavior and position of the elements present in the periodic table and how they form chemical bonds with each other (Singh, 2011).
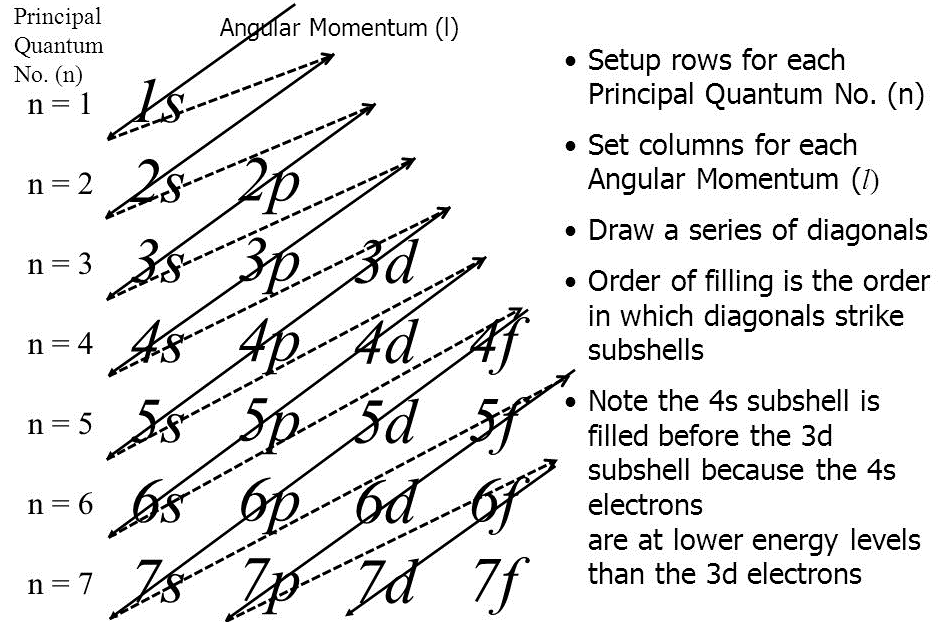
It can be written in the following formats:
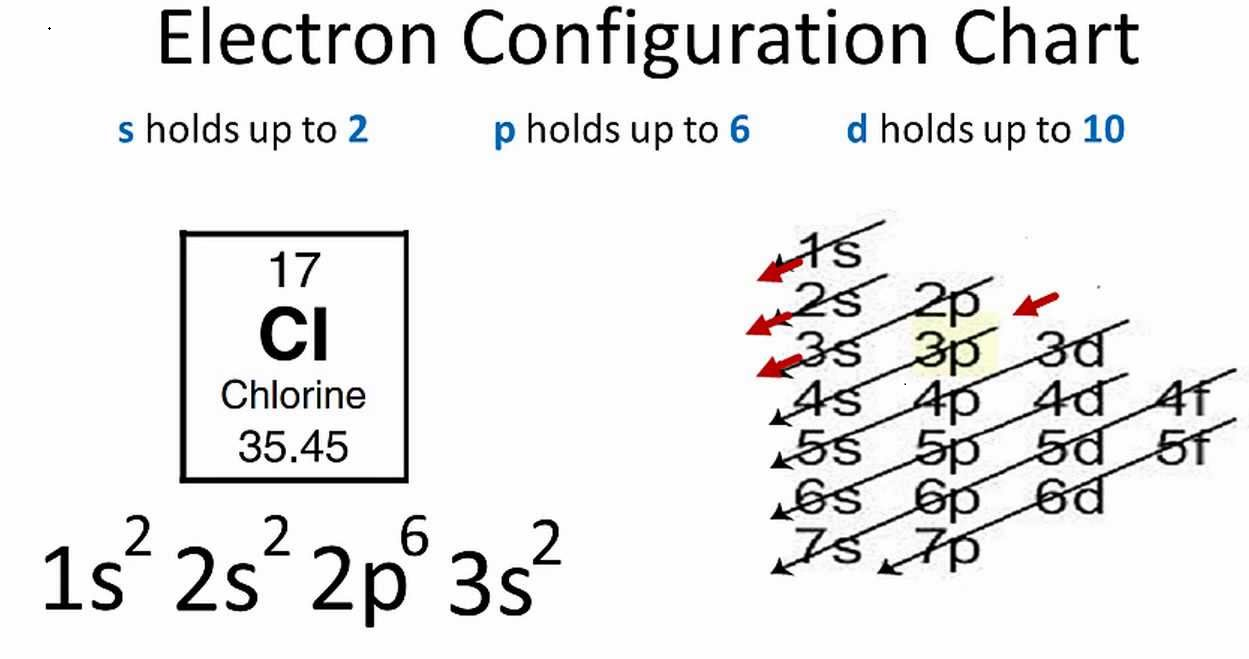
- spdf notation: e.g, Cl(17)= 1s22s22p63s23p5
- Orbital diagram: e.g, Cl(17)=
- Inert gas/condensed notation: e.g, Cl(17)= [Ne]3s23p5
- Valence electron configuration: e.g, Cl(17)= 3p5
- Shell notation: e.g Cl(17)=2, 8, 7
In the first notation, the shell (k,l,m,n…) is represented by a number (1,2,3,4…), subshells (l) are represented by s,p,d, and so no. The superscript represents a number of electrons in a respective subshell. In the second notation, each box represents an orbital where the electrons are drawn with a positive spin (↑) or negative spin (↓). The second notation represents all the four quantum numbers. The other formats are a variation of spdf notation. For instance, valence electrons are present in the outermost shell and they signify the chemical properties of an element.
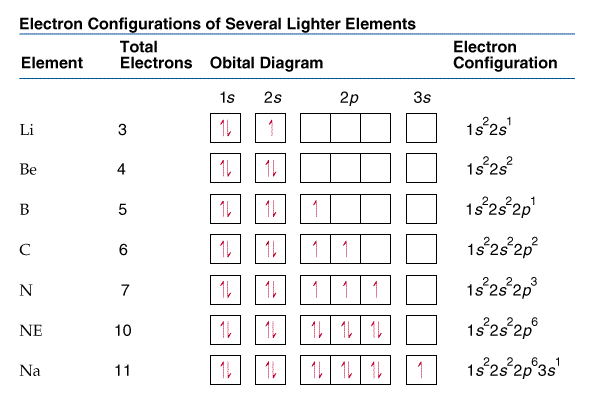
Figure 2: Electron configuration of lighter elements.
Hence, the general order in which the electrons occupy orbitals is defined by Aufbau’s principle, Pauli’s exclusion principle and Hund’s rule whereby discrete energy levels, shape and spin segregates one electron from another.
| Shell | Subshell | Orbital |
| Shell is the pathway followed by electrons around an atom’s nucleus | A subshell is a pathway in which an electron can move within a shell | A mathematical function that describes the wave-like the behaviour of electron |
| It is defined by principal quantum number ‘n’ | It is defined by angular momentum number ‘l’ | It is defined by a magnetic quantum number |
| A shell can hold up to 32 electrons. | s subshell can hold 2 electrons, p subshell can hold 6 electrons, d subshell can hold 10 electrons, f subshell can hold 14 electrons | An orbital can hold up to 2 electrons in a positive or negative spin. |
In conclusion, a maximum number of electrons can be obtained by 2n2 where ‘n’ is the number of shells (1, 2, 3…). Also, electron occupies the higher shell only after filling up the lower energy shell. Within a subshell, all the orbitals are degenerate, therefore electrons will not pair up until all orbitals have single occupancy within the subshell. Moreover, when two subshells have a similar energy level, electrons may prefer to obtain a half/completely filled shell instead of a lower energy shell. Finally, the maximum number of electrons in the outermost shell (valence shell) cannot be more than 8 (Huheey, Keiter, Keiter, & Medhi, 2006).