Atoms are the fundamental building blocks of the matter. Atoms are extremely small in size; typical its sizes are around 100 Pico meters . Every solid, liquid, gas, is composed of neutral or ionized atoms. Every atom has a nucleus and one or more electrons bound to the nucleus.
| Protons——————— Positive charge;
Neutrons——————–No charge; Electrons———————- Negative charge. |
Structure of an Atom: Structure of an atom can be basically divided into two parts:
- an atomic nucleus
- extra nucleus part
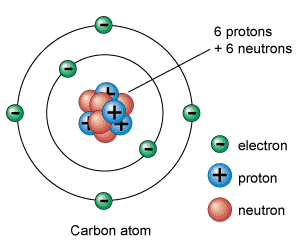
The nucleus of an atom is made of one or more protons and typically a similar number of neutrons. Protons and neutrons of an atom are together called as nucleons . If the number of protons equals the number of electrons, then that atom is electrically neutral. An atom is called an ion if it has more or fewer electrons than protons, also it will have an overall negative or positive charge, respectively. The number of protons in the nucleus decides to what chemical element the atom belongs. The isotope of an element is defined by number of neutrons. The number of electrons in an atom influences its magnetic properties.
The electrons of an atom are attracted to the protons in the nucleus by the electromagnetic force. The protons and neutrons residing in the nucleus are attracted to each other by a different force, the nuclear force, which is usually stronger than the electromagnetic force.
Atomic Number: The atomic number is the total number of protons present in the nucleus of an atom. It is denoted by Z.
Mass Number: The mass number is the sum of the total number of protons and neutrons present in the nucleus of an atom.
Isotopes: Isotopes are the atoms of the same element, having the same atomic number but different mass numbers. The three isotopes of hydrogen atom are namely protium, deuterium and tritium.
Isobars: Isobars are defined as atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars.
Valency: Valence electrons are basically the electrons present in the outermost shell of an atom. The atoms of elements which have a completely filled outermost shell show little chemical activity i.e. their combining capacity or valency is zero. An atom of each element has a definite combining capacity which is called its valency.
Atomic Models:
There are some basic atomic models which have contributed the structure of the atom itself.
- John Dalton’s atomic model: The 4 mains points of his model are:
- All matter is made of atoms.
- Atoms are indivisible and indestructible.
- Compounds are formed through the combination of two or more different kinds of atoms.
- A chemical reaction is a rearrangement of atoms.
- J.J. Thomson’s model: an atom resembles a sphere of positive charge with electrons present inside the sphere. The positive and negative charge is equal in magnitude and therefore an atom has no charge as a whole and is electrically neutral.
- Bohr’s Model: Neils Bohr proposed a theory for the hydrogen atom based on quantum theory that energy is transferred only in certain well defined quantities. Electrons can move around the nucleus only in prescribed orbits. When electrons jump from one orbit to another, a light quantum is emitted.