An atom has an equal number of protons and electrons which means that it is electrically neutral. However, in a chemical reaction, atoms tend to either gain or lose electrons forming an ion. An ion can be defined as an atom with either a positive or a negative charge. The ionic radius is the effective distance from the centre of the nucleus of an ion up to the point it has an influence on its electron cloud.
Electromagnetic interaction such as shielding and penetration dictate the orbital characteristics of ions. Hence s-orbital electrons can penetrate and shield more effectively than p- orbital electrons. Likewise, p-orbital electrons penetrate and shield more effectively than d-orbital electrons. Therefore, the nuclear charge in addition to shielding and penetration determines the size of an ion.
The effective nuclear charge can be defined by the following equation:
Where
Z is the atomic number, and
S is the number of electrons in the core of an atom
For metallic elements, atoms lose one or more electrons and form positive ions called cation. A cation has fewer electrons than an electrically neutral atom. For example a sodium atom, Na has 11 electrons while its cation has only 10 electrons making its net charge +1. Due to the loss of outer shell electrons, cations are smaller than atoms as less electron-electron repulsion allows the electron and nucleus to come closer to each other. Therefore a sodium cation is smaller in size than a sodium neutral atom.
For non-metallic elements, atoms gain one or more electrons to form negative ions called anion. This means that an anion has more electrons than an electrically neutral atom. For example, a fluorine atom, F has 17 electrons while it’s an anion gains one more electron and makes its net charge of -1. Due to the gain of electrons in the outer shell, the anions are larger in size than neutral atoms. The electron-electron repulsion increases and the attraction between the nucleus and the remaining electrons decreases which causes the electron cloud to spread apart. For example, a fluoride ion, F– is larger in size than a neutral atom of fluorine, F.
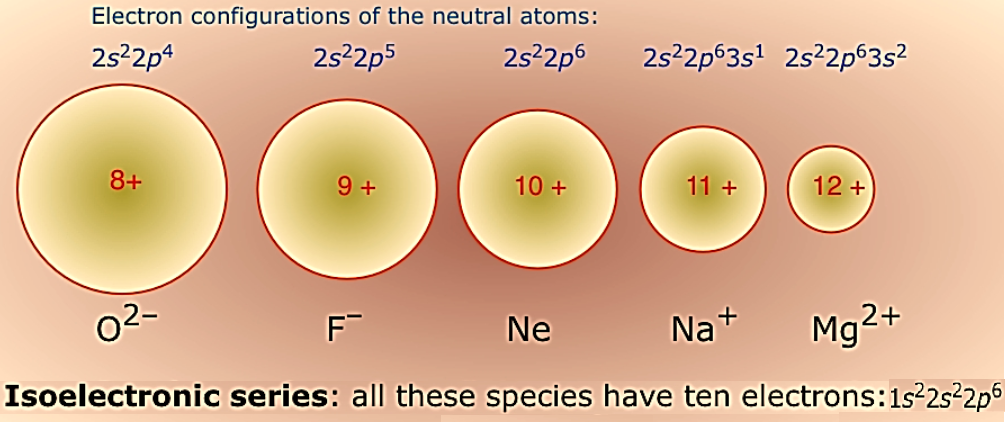
Figure 1: Ionic radii of isoelectronic species.
In the case of isoelectronic species, a cation has a smaller radius with a greater positive charge but anion has a larger radius. For example, O2–, F–, Na+ and Mg2+ have the same number of electrons. Since the nuclear charge is different, cations will have smaller radius due to higher positive charge and greater attraction between nucleus and electrons. However, anion has a larger radius since the net repulsion of electrons is more than the nuclear charge.
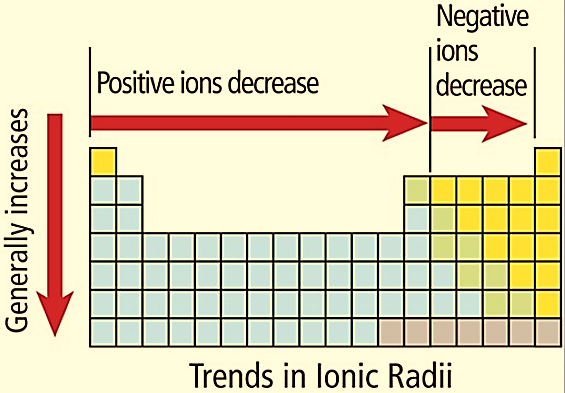
Figure 2: Periodic trends – Ionic Radii
As we go down the group, the ionic radius increases since each row adds a new electron shell to the ion. As we go from left to right across a period, the ionic radius decreases since the positively charged nucleus draws the electrons closer to each other. In non-metallic elements, the ionic radius increases across the period since there are more electrons than protons.