When an electron is added to an isolated gaseous atom or ion in its ground state molar enthalpy change is certain which is called the electron gain enthalpy. Electron Gain Enthalpy is completely different to the Ionization enthalpy. For example, the halogens have highly negative electron gain enthalpy because they cannot obtain a stable noble gas electronic configuration by adding another electron in the outermost shell. Alternatively, noble gases have highly positive electron gain enthalpy because the electron would have to enter the next principal quantum level to become an ion. Larger negative values of electron gain enthalpy are observed in the upper right corner of the periodic table preceding the noble gases.
These factors influence the Electron gain enthalpy:
1) Atomic Size
As the size of the atom increases, the distance between the nucleus and the outermost shell increases. This decreases the attraction between the nucleus and the incoming electron. Hence, the electron gain enthalpy becomes less negative.
2) Nuclear charge
As the nuclear charge increases, the attraction between the nucleus and the incoming electron increases. Hence, the enthalpy becomes more negative. As we move down to another group, atomic size and the nuclear charge increases together. But the effect of the increase in atomic size is more than the nuclear charge.
3) Electronic configuration
Elements with exactly 1/2 or full filled orbitals are purely stable. Or , their electron gain enthalpy has large positive values.
4) Type of sub-shell where the incoming electron is being added
Electrons are packed most tightly in s sub-shell as it is the smallest one. Since p-subshell holds more space for electrons than s, so it is easier to insert an extra electron in p subshell than in s subshell. In a larger subshell, the incoming electron must face lesser repulsion from other electrons. The order of the electron gain enthalpy for the sub-shells is s > p > d > f
The electron gain enthalpy becomes more negative from left to right in the period and less negative from top to bottom in a group. Once we head to a period from left to right the atomic size gets decreasing and the nuclear charge increases indeed. This happens because it is easier to add an electron to a smaller atom as on average as electron will be closer to the nucleus. As the size of an atom increase, the electron moves further away from the nucleus.
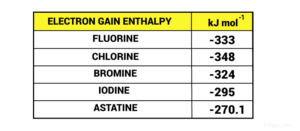
Figure 1: Periodic trends – Electron gain enthalpy of halogens
Chlorine has the most negative electron gain enthalpy. The electron gain enthalpy of fluorine is less negative than chlorine owing to a smaller size where the electron-electron repulsion is comparatively smaller. Hence, the incoming electron is not accepted with the same ease as is the case with chlorine. The elements of the second period have the smallest atomic size among the elements in their respective group. As an outcome, there are noticeable electron-electron repulsions within the atom itself indeed. The additional electron is not accepted with the same ease as is the case with the remaining elements in the same group.