Under standard conditions, noble gases are colorless, odourless, tasteless and nonflammable. The outer shell of valence electrons is considered full in noble gases making them chemically non-reactive and they also happened to have the largest ionization energy. The general configuration of the valence shell is ns2np6 except helium [He]: 1s2
Since the noble gases are monatomic, their non-bonded radii values are very large. Therefore, its radius is measured using Van der Waal’s method. Van der Waal’s radius is measured by taking half of the internuclear separation between two atoms of the same element adjacent to each other in their solid state.
In general terms, Van der Waal’s force defines any attractive intermolecular force between molecules. Van Der Waal’s include both Landon dispersion force and dipole-dipole force. These are the only intermolecular force that works on noble gases and nonpolar molecules. The Van der Waal’s radii are naturally higher than the covalent radii of other elements such as halogens.
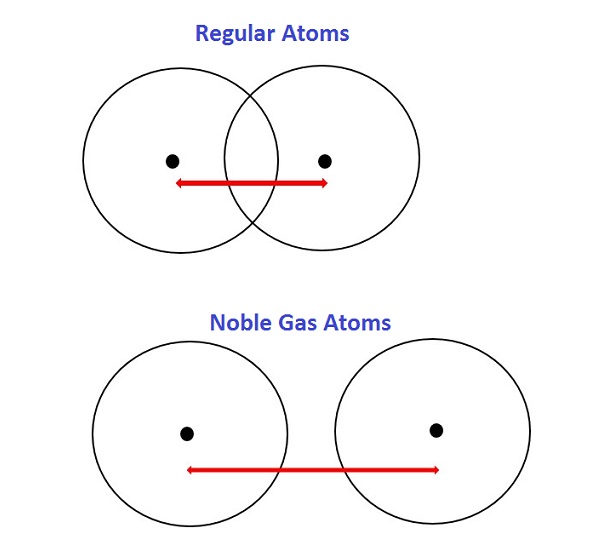
Figure 1: Difference between the atomic size of other elements and nobel gases.
As we go down the group, the Van Der Waal forces within the atoms the increases and the inter-atomic forces increase which causes the atomic size to increase. Electrons in bigger noble gases move away from the nucleus which means that has less tightly by the nucleus. The gases also get denser as the atomic number increases. Increase in Van der walls forces also causes the melting point and the boiling point to increase in addition the ease of liquidation also increases. When the noble gases condense, the intermolecular forces that hold the liquid together are induced dipole forces.
The size of atoms abruptly increases as we move from halogens to inert gases. Because the Interatomic electronic force in inert gases is more as compared to the halogens completely filled orbitals since the atomic size is expressed in terms of Van Der Waals radius. The noble gas atoms look bigger, even though they are not. Therefore, noble gas radiiare not accurate to understand how big noble gas atoms really are. Because noble gas atoms bond differently, their radii can’t be compared to the radii of other atoms, so they don’t follow general atomic radius trends.
Gases down the group are held together less strongly by electromagnetic forces then lie to noble gases such as helium. Out of the six noble gases, Krypton, Xenon and Radon can form stable compounds which are good oxidizing agents.
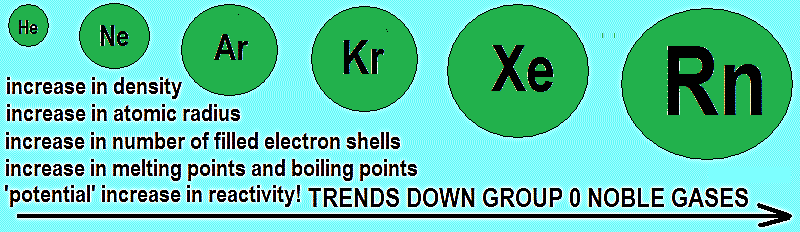
Figure 2: Noble gases
Helium is chemically a highly unreactive element. It only forms transient species when electric discharges are passed through a mixture of helium gas and another gaseous element. Neon is chemically the most unreactive element. Argon, Xenon and Krypton clathrates but most of these compounds are unstable.
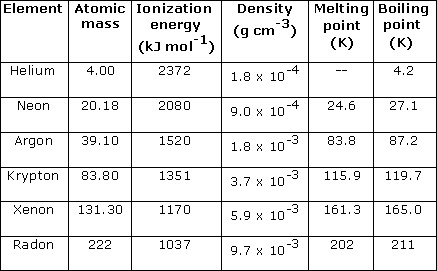
Figure 3: Properties of noble gases
Indecently, atomic size decreases along a period, therefore, the atomic radius of Ar should be smaller than Cl. But, noble gases are monoatomic with Van der Waal’s forces instead of covalent bonds between atoms. Since the Van der Waals radii are bigger than covalent radii, the atomic radius of Argon is larger than Chlorine.