Atom is composed of various sub atomic particles carrying positive or negative charge as observed and experimented by various scientists. Soon after these sub particles were discovered, scientists became eager to explain the distribution of these charged particles in an atom. It was major challenge before the scientists at the end of the 19th century to reveal the structure of the atom as well as to explain its important properties. For this different atomic models were proposed. One such model was proposed by J.J Thomson.
J.J. Thomson was a British physicist, and was born in Cheetham Hill, a suburb of Manchester, on 18 December 1856. For his work on the discovery of electrons he was awarded the Nobel Prize in Physics in 1906. An Electron i.e. a negatively charged particle was discovered by J.J. Thomson during cathode ray tube experiment. Cathode ray tube is a vacuum tube. The negative particle was called an electron. This experiment took place in the year 1897.
The model of an atom proposed by Thomson was compared with a Christmas pudding by him. In a sphere of positive charge, the electrons were like currants (dry fruits) in a spherical Christmas pudding. We may also think link this to a watermelon, in which the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are embedded in the positively charged sphere, exactly like the seeds in the watermelon.
He believed that an atom is made up of thousands of electrons and assumed that an electron is two thousand times lighter than a proton. In his atomic structure model, he considered atoms to be surrounded by a cloud having positive as well as negative charges. Thomson was successful is explaining the overall neutrality of an atom.
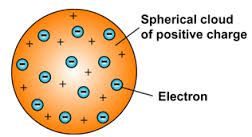
Thomson’s atomic model states that:
- An atom consists of a positively charged sphere with electrons embedded into it.
- Both the negative and positive charges are equal in magnitude. This makes the atom as a whole to be electrically neutral.
It must be noted here that in this model the mass of the atom is assumed to be uniformly distributed over the atom. Thomson also demonstrated the ionization of air by X-ray along with Rutherford. They were the first ones to demonstrate it.
Limitations of Thomson’s atomic model
- It failed to explain the stability of an atom because his model of atom failed to explain how a positive charge holds the negatively charged electrons in an atom. Therefore, this theory also failed to tell us about the position of the nucleus in an atom
- Thomson’s model failed to explain how thin metal foils can cause the scattering of alpha particles.
- These is no experimental evidence in its support
Thomson’s model proved to be the base for the development of other atomic models even though it is not an accurate model to account for the atomic structure. The study of the atom as well as its structure has paved the way for numerous other inventions that have played a significant role in the development of mankind.