Three States of Matter
While everything in this universe is known to be composed of matter, studying this concept further is worthy.
What is Matter?
Anything that is visible and tangible or invisible but could be felt and has an existence is known to be composed of matter. Based on the varied inter-molecular forces and the arrangement of particles, matter is known to exist in three major forms: solid, liquid and gas. The state of matter could be changed by changing temperature and decreasing or increasing pressure. With the change in the state of matter, the arrangement of particles and structure is said to be altered.
To elaborate this, consider several different things around you. There are different forms in which things would exist in the nature. While some substances are rigid with a fixed shape like wood, there are others in fluid form that can flow like water. There are more that do not have a definite shape of their own; these prevail in the gaseous form.
States of Matter
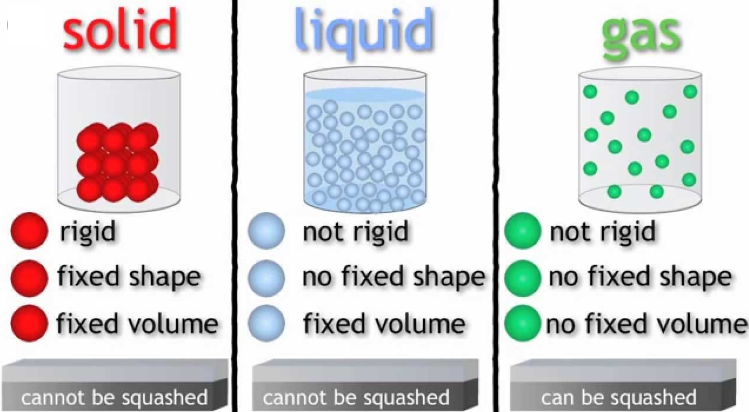
The above introduction implies that based on different physical properties, matter could be separated into several different states. Here are a few deeper insights into the different states of matter: solid, liquid and gas.
Solid State
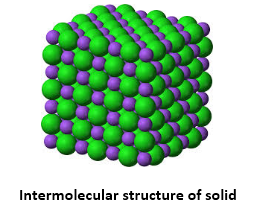
One among the major states of matter is the solid state. Rigidity is the major characteristic that separates solids from liquids and gases. The structure for matter in solid form includes tightly packed molecules with comparatively stronger intermolecular spaces.
The molecules in a solid structure are known to oscillate only in their mean structure. However, for liquids and gases, this is a bit different case with fluidity. Solid state of matter is known for possessing definite shape, volume and rigidity. They also possess least thermal expansion and compressibility. Example: Iron
Liquid State
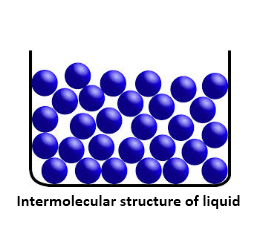
Because of weak intermolecular forces, molecules in a liquid state matter are known to be closely packed. These forces are weaker than the solids however, stronger that the gases. The greater spaces between the molecules make it easy for liquids to flow. Liquids tend to have a fixed volume and easily acquire the shape of the vessels they are poured into.
The increase in temperature of the solids till their melting points would lead to their conversion to liquids. The density of liquids, range in between the solids and the gases. As compared to the solids, liquids offer more thermal expansion and compressibility. Example: Water, Sulphuric Acid.
Gaseous State
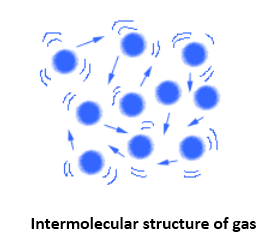
In the gaseous state, the molecular distances are known to be too large. Also, the intermolecular forces among these are almost negligible. Gaseous matter therefore showcase, vibratory, rotatory and translator movement. Gases do not have any fixed volume or shapes. Also, they offer much higher compressibility and thermal expansion as compared to any other states. Example: Oxygen
Knowing more about the different states of matter is worthy to carry on chemical reactions perfectly further. It becomes sophisticated to handle different elements when you know well about their state and respective properties. Fundamentally, matter would generally prevail in solid, liquid and gaseous forms.