Today, it is a well-known fact that the atomic number reveals the number of protons in the nucleus. This was discovered by Henry Gwyn-Jefferies Moseley. He conducted the experiments and found certain lines in the spectrum of X-ray of each element and each time it moved the same amount by an increase in the atomic number as one.
By describing the results of his research, he stated that here it is the proof that atom is a fundamental quantity that is increased by the regular steps, as we proceed from the one element to the next element. This quantity is only changed due to a change in the central positively charged particles in the nucleus and the existence of protons in the center of the nucleus has already been proved. Moseley was the part of the research group of Rutherford. He started working on the concept of the atomic number just after the publication of Rutherford’s paper on the nucleus. Just after a few months of publishing Rutherford’s paper the physical meaning of atomic number was truly suggested in 1913 by A. Van Den Broek.
Earlier, Rutherford was not excited that Moseley wanted to study the X-rays but later on, with his enthusiasm and energy, he convinced Rutherford and soon he was down for Moseley’s passion. The main study purpose of Moseley was to find out the linear relationship between the atomic number and measurable property of the nucleus. The atomic number is increased by one step and the same condition is applied to all of the elements. He was looking for some function of nuclear property that is increasing by using the same pattern i.e. one for each of the elements. According to the results, the square root of frequency moves with a constant value for each of one unit move of the atomic number.
Moseley Study Made an Impact for Discovery of Atomic Number
Moseley has chosen to study the X-rays it was demonstrated by the Charles Barkla that characteristics X-rays are emitted by the elements known as K rays and L rays and are independent of the chemical or physical state of the element. Then it was realized that these X-rays were the characteristics of the nucleus. So, Moseley determined wavelengths of K radiation by using techniques that were recently discovered by W.H. Bragg and W. L. Bragg. At that time Moseley was much confident as he had to find out the linear relationship in a proper way. Finding the right working equipment for producing reliable data was a time-consuming task throughout his entire research. However, in the end, he was able to find out the relationship and this relation was linear with the square root of the frequency value and moving upward in the same amount with the increase of each one unit in the atomic number.
Moseley’s Graph of Atomic Number
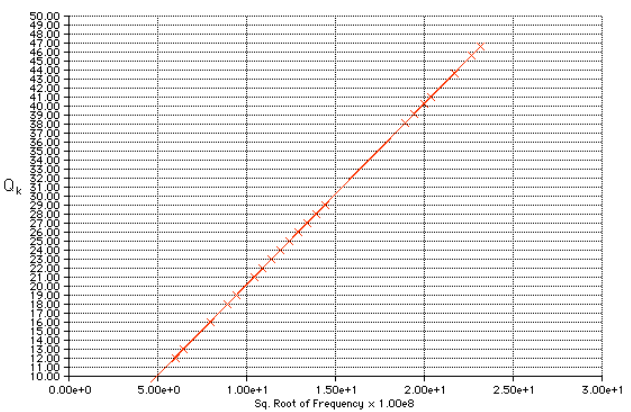
It is the graph obtained by the Moseley’s data that shows the linear behavior. In this way, he proved that the atomic number is the number of protons present in the nucleus of the atom. In other words, it can be said that the atomic number is the same as the number of positive charges in the atom.