Some types of shapes are found in molecules of different substances. Also, these can be based on the study and the understanding of their properties.
Linear Molecules
The highlighting feature regarding linear molecules is – there’s repulsion acting mutually regarding all electron groups which are present so that their atoms are aligned to a straight line, in which there is a bond angle of 180 degrees.
Examples of a linear molecule including all diatomic molecules:
H2 → H — H
Cl2 → Cl — Cl
O2 → O — O
Also, specific there are linear polyatomic molecules:
CO2 → O = C = O
Ethyne, C2H2 → H – C ≡ C – H
Non – linear Molecules
The repulsion between the acting electron groups is not exactly the same in the case of non-linear molecules. It has been found that repulsion between the bonding pair and a lone pair is higher than between the two bonding.
This is the reason why H2O molecule has a bent structure and the trigonal planar or triangular pyramidal shape of NH3 (Ammonia) molecule.
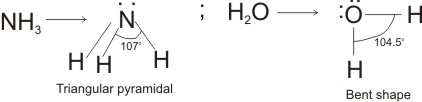
It’s important to note that the angle created between the two bond pairs in NH3 is greater (107o) than the H2O molecule, having 104.5-degree angle. This is because of a larger number of loan pairs acting on the oxygen of H2O, which make use of maximum repulsion on bonding pairs.
Trigonal pyramidal:
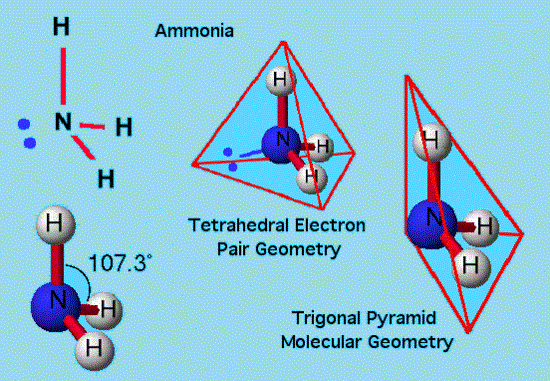
The triangular pyramid molecule consists of a pyramid-like shape accompanying a triangular base. Dissimilar to trigonal planar and linear shapes but identical to Tetrahedral Orientation/location, the pyramid-shape like structures require 3 dimensions so as to completely isolate the electrons.
In this, there are only 3 bonded electrons’ pairs, which leave one lone pair. Bond-pair – Loan pair repulsions transforms bong angle to marginally lower value from a tetrahedral angle. Let’s say, ammonia (NH3).
Trigonal planar:
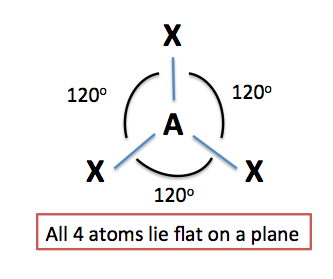
The molecules having triangular planer shape are considerably triangular and align in one plane (flat). As a result, the bond angles are formed at 120 degrees. For instance, boron trifluoride.
Angular Molecules:
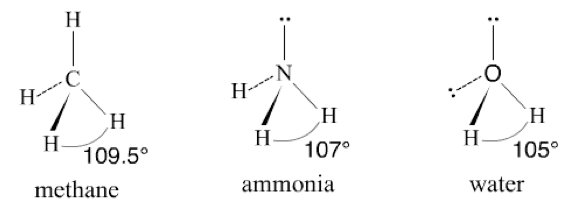
Angular Molecule is also called, V-shaped or bent molecule, which consists of non-linear shape. For instance, an H2O molecule is having an angle of approximately 105 degrees. A water molecule consists of two bonded electrons’ pairs and two lone pairs that are unshared.
Tetrahedral Molecules
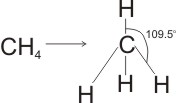
The result of tetrahedral molecules is when the tetravalent atoms (such as carbon {C} and silicon {S}) pass through absolute covalency accompanying atoms of the same element. 4 covalent bonds are dispersed in 3 dimensions in a symmetrical manner, and the angle between any two bonding pairs is 109.5 degree.
According to VSEPR (Valence-Shell Electron-Pair Replacement Concept), bond angles between the electron bonds are set at 109.47 °. For instance, methane (CH4) is a tetrahedral molecule.
You can see in the examples of tetrachloromethane (CCl4 ); methane (CH4); Silane (SiH4)
Octahedral:
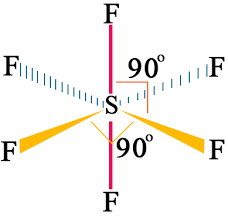
Octa- means eight, and -hedral defines the face of a solid, thus, “octahedral” signifies “consisting of eight faces”. The bond angle is set at 90 degrees. For instance, (SF6) sulfur hexafluoride is an octahedral molecule.