Types of bonding
The formation of Chemical compounds is via connection of two or more than atoms. A stable compound arises when the entire energy of the mixture has lesser energy than isolated atoms. Bound state indicates a purely attractive force between atoms. The two out most conditions of chemical bonds are:
-
- Ionic Bond
- Covalent Bond
- Polar covalent bonds
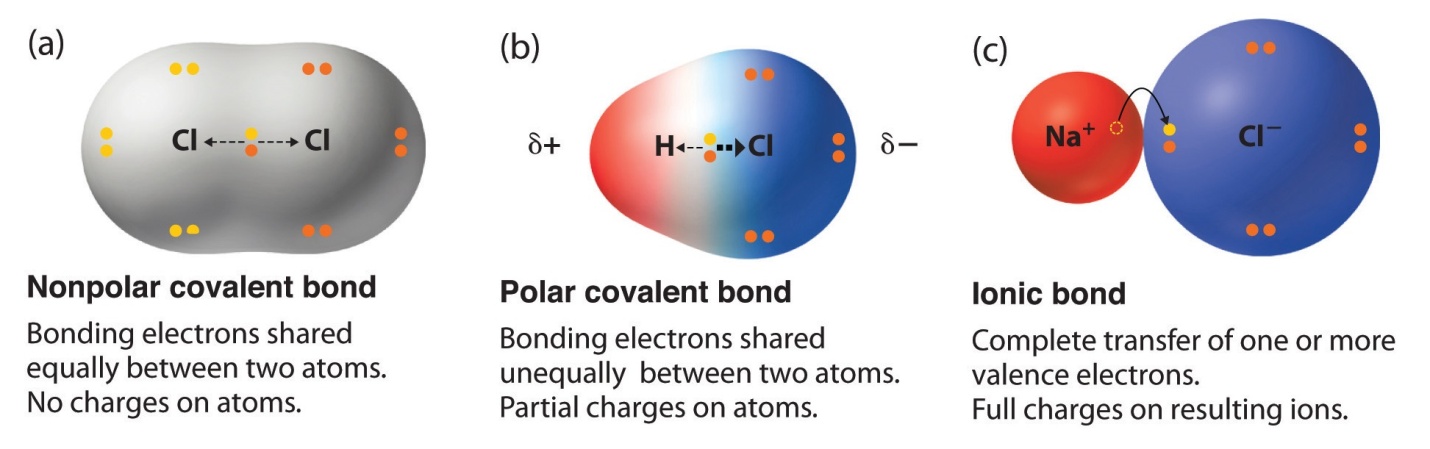
Key Points to remember:
Various types of bonds can be formed in non-metals which depends on their fellow atoms. Ionic bonds are formed when one metal and a non-metal tends to exchange electrons. On the other hand, the covalent bond is formed when the electrons are shared between 2 non-metals.
An electron pair is involved in a covalent bond which is shared in between the atoms.
Covalent bonds are formed by atoms in order to achieve a greater extent stable state.
The single, double, or triple bond is formed by a non-metal with another non-metal present in it. On the basis of no. of valence electrons, it’s dependent that which bond type is formed between the acting atoms.
Ionic Bonds
The Ionic bond is referred to as a type of chemical bond that is formed through electrostatic attraction between two charged ions which are opposite in nature. The formation of ionic bonds takes place between an anion which is generally a non-metal and a cation which is mostly metal.
A pure ionic bond can’t exist: A whole of the ionic compounds consist of some degree of a covalent bond.
Thus, the ionic bond is observed as a bond in which the ionic character is more than a covalent character. The greater the electronegativity difference between two atoms included in bonding, the more will be the ionic or the polar bond.
Partially covalent and ionic character are known to be Polar Covalent Bonds.
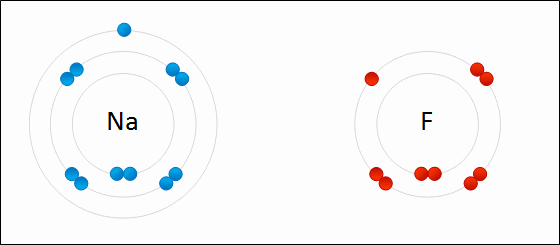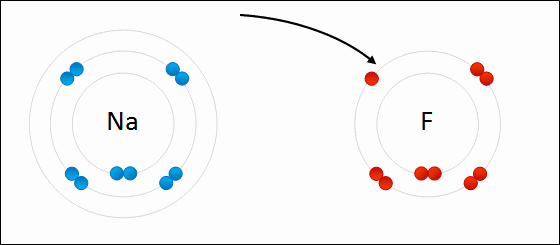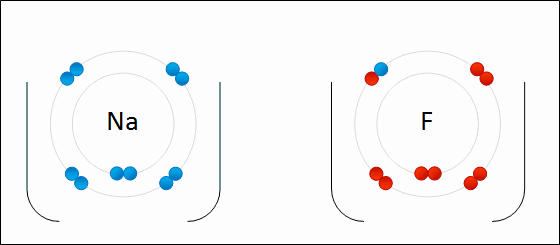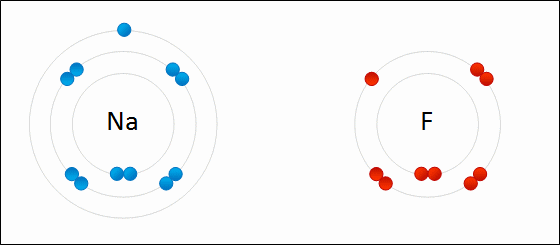
Sodium Flouride Formation- the Ionic compound is formed when there is an attraction between oppositely charged atoms plus the transfer of electrons.
Covalent Bonds
Covalent bonds are formed when the electrons are shared between the atoms. Atoms’ best stable state takes place when the shell of its valence electron is full/complete, thus, the atom creates a covalent bond by sharing their valence electrons, in order to get an additional stable state by filling their shell with valence electron.
Polar covalent bonds
There is a small difference in the charge with one direction of a molecule of a few covalently bonded compounds. The dissimilarity in charge is known as a dipole and when with this difference in charge, covalent bond forms, the bond is known as polar covalent bond.
Such bond formation takes place when shared electrons are not evenly shared between the atoms. If in case, one atom possesses higher electronegativity, the electrons will be more attracted towards the nucleus of that particular atom, which results in a slight net charge throughout each nucleus of atoms in a molecule.
If atom in a molecules features the same electronegativity (let’s say, if atoms are equal, as in case of N2), then shared electrons will not be attracted towards more than one nucleus, and the bond formed will be nonpolar.
Likewise, the greater is the electronegativity difference, more unequal sharing of electrons will be found between the nuclei, and higher will be the polarity of the bond.
