What is Boiling Point?
The boiling point exists as the temperature at which liquid’s vapor pressure is equivalent to liquid’s atmospheric pressure of the liquid and then the liquid change its state to vapor form.
As soon as the liquid starts boiling, the temperature remains constant until all of the liquid gets converted into a gas.
Liquid’s boiling point is dependent on the neighboring pressure. Liquid at higher pressure contains higher boiling point comparative to the boiling point present at the usual atmospheric pressure. For a given amount of pressure, for different liquids, the boiling point is also different.
In 1982, IUPAC described the standard boiling point, which refers to the temperature at which boiling of liquid takes place having the condition of 1 bar pressure. The boiling point varies with height and this is the reason why when people go to hilly areas, that is, it takes longer time to cook at high altitude because the pressure decreases and due to this it takes more time to cook meals in mountainous areas.
What is the Melting Point?
The point at which solid state changes into the liquid state at atmospheric pressure which is known as the melting point of that particular liquid. It’s that point at which both solid and liquid phase is at the equilibrium state. The melting point of matter also changes with pressure and is clearly identified on standard pressure.
Another word is the freezing point which is exactly opposite to the melting point, which is the temperature on which the liquid is converted into a solid state. So, technically, it can be understood that the freezing point and melting point are different as the substance can be immensely cooled below its freezing point without forming into solid.
If not the case of impossible, then it’s difficult to heat solid above its melting point as the heat which enters into the solid at its melting point is used for conversion of solid into a liquid. However, it’s possible to cool a few of the liquids at temperatures below their freezing points with the formation of solid. And then the liquid is known to be supercooled.
Measuring the melting point of a solid can even provide information regarding the purity of the substance. The crystalline, pure solids melt above a very narrow temperature range, although the mixture melts at a wide temperature range. The mixture also melts at temperatures below the melting points of the pure solids.
Now let’s see the melting and boiling points of various substances:
| Name of the substance | Boiling point(K) | Melting point(K) |
| Aluminum | 2740 | 932 |
| Copper | 1460 | 1359 |
| Gold | 2933 | 1336 |
| Hydrogen | 20.3 | 13.8 |
| Mercury | 630 | 234 |
Boiling Point
In the concept, you should not be able to heat the liquid at the temperature above its regular boiling point. Before the time of microwave oven, nonetheless, the pressure cooker was in use for reducing the cooking time.
In a conventional pressure cooker, the water can remain liquid at a high temperature of 120-degree Celsius, and the food cooking takes one-third less time than the normal cooking time.
To explain why water boils at 90oC in the mountains and 120oC in a pressure cooker, even though the normal boiling point of water is 100oC, we have to understand why a liquid boils. By Explanation, a liquid boils that time when the vapor pressure of the gas is break free from control from the liquid is equal to the pressure exerted on the liquid by surroundings, as shown below.
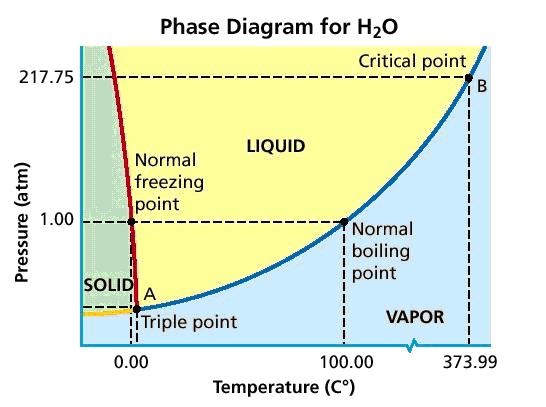
100oC is the water’s normal boiling point as this is the temperature at which you’ll find water’s vapor pressure at 1 atm or 760 mmHg. And above sea level which is at 10,000 feet, the atmosphere’s pressure is only 526 mmHg.