Chemical Thermodynamics: Surroundings
A system is a part of the universe in which we make observations while all the remaining part of the universe is called surrounding. We can consider the ‘Surrounding’ as the immediate neighborhood of the system (the part of the universe at large, with which the system ‘effectively’ interacts). In this process of things we can imagine: a system, the surrounding and the universe at large base. A bird is a system which is separated from its surrounding by its skin and feathers. The bird can exchange both energy and matter with its environment. If Earth is considered as a system, everything around it such as the atmosphere and outer space are all its surrounding. In this case, the earth can exchange energy with the environment but not matter.
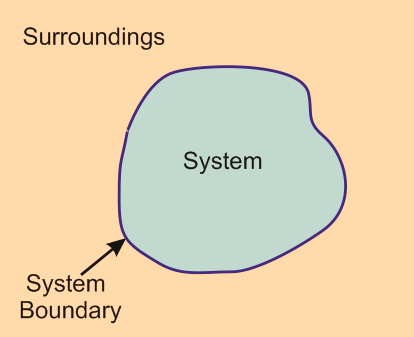
Figure : A thermodynamic system is surrounded by a boundary and surrounding within a universe.
A boundary separates the system and surroundings. The boundary does not have any thickness or mass or volume Fixed boundary eg. a rigid box containing gas. Movable boundary eg. Cylinder with the piston. Therefore, it is critically important to define a system with its boundary and surroundings to solve any thermodynamic system. The boundary of an open system is known as a control surface which can either be real or imaginary. A closed system has moving boundary while an isolated system has a real and fixed boundary
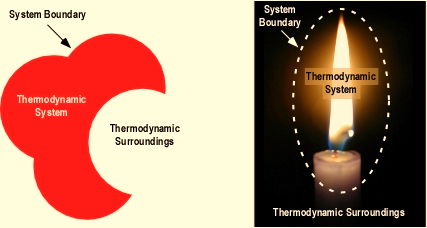
Figure 2: An imaginary boundary surrounding a candle to separate it from the surrounding air.
For example, if you’re studying the reaction between two substances A&B placed in a beaker. the beaker containing the reaction mixture is the system and the room where the beaker is the place is the surrounding. The system is defined by physical properties like the beaker or test tube. It can also be defined using Cartesian coordinates specifying a particular volume in space. it is necessary to understand that the system is separated from the surrounding by a boundary that may be real or imaginary. this boundary is designed to allow us to control and keep a track on an all the movements taking place in and out of the system with respect to matter and energy. For example, when a cube of dry ice diffuses into the air without melting at the temperature of -78.5 degrees Celsius, a definite boundary cannot be specified.
Table 1: Types of boundaries
| Type of boundary | Interactions |
| Open | All interactions possible (Mass, Work, Heat) |
| Closed | Matter cannot enter or leave |
| Semi-permeable | Only certain species can enter or leave |
| Insulated | Heat cannot enter or leave |
| Rigid | Mechanical work cannot be done |
| Isolated | No interactions are possible |
Hence, in general terms the universe includes everything but we will mostly look at things on a smaller scale. For example, consider a burning fuel package as the system and the compartment as the surroundings. On a larger scale, the building containing the fire as the system and the exterior environment as the surroundings is another example.