Chemical Thermodynamics: Energy
Every system or sample of matter has energy stored in it. In a chemical reaction, the new bonds formed never have the same amount of potential energy as the bonds that were broken. Therefore, all chemical reactions involve a change in energy. Energy is either given off or taken on by the reaction.
The SI unit of energy is the Joule (J):
Energy is often measured in other units, specific to applications. These include
- Nuclear Energy – The special energy needed to bind nucleons in the nucleus
- Light Energy – The potential energy possessed by the oscillating electric and magnetic fields that make up electromagnetic radiation
- Chemical Energy – The potential energy stored in the electrostatic bonding relationships among atoms in a molecule
- Electrical Energy – The special energy used in initiating and sustaining electron flow
- Mechanical Energy – The energy generated (or stored) by machines which induce (or results from) concerted motion processes in a system
- Heat Energy – The kinetic energy associated with the random motion of matter including the vibratory and rotatory action of molecules
Internal energy is the sum of all types of energies of the molecules which is very difficult to calculate. Total energy possessed by any system due to its molecular configuration and motion is called internal energy. It depends on temperature and it is a characteristic property of the state of a system. The energy possessed by molecules, the arrangement and number of electrons in the molecules, all contribute to internal energy. In addition, the energy due to the vibrational, rotational and translational motions of the molecules is also included in internal energy. Chemical and physical changes are accompanied by rearrangements in the relative positions of the atoms. This leads to changes in internal energy. Currently, it is impossible to determine the absolute value of the internal energy of a system. However, chemists are interested only in it is the change in internal energy accompanying a chemical or physical change.
For instance, a system containing calcium oxide and water has some internal energy in the form of chemical energy at the beginning of the reaction. As the reaction proceeds and reactants change into calcium hydroxide, a large amount of heat energy is evolved. As an outcome, the insider energy of the system reduces. The change in energy can be calculated by subtracting the initial state from the final state. The insider energy of a system up on the state of the system not the path.
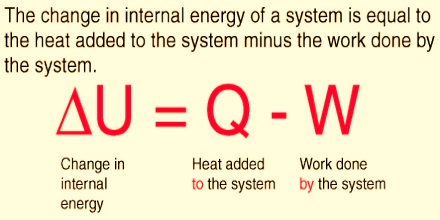
Figure 1: Change in energy
The internal energy of an isolated system remains constant since no interactions in the form of heat or work are done on or by the system. For a closed system, interaction with the surroundings are allowed, energy is transferred from system to surrounding or vice versa in the form of heat and/or work. In this case, the total energy of a system and surrounding is preserved.
Table 1: Relationship between energy, heat and work
| Property | Positive sign | Negative sign |
| ∆U | Net gain of energy by the system | Net loss of energy by the system |
| W | Work was done on the system | Work is done by the system |
| q | The system gains heat: endothermic | The system loses heat: exothermic |