The discovery of isobars was a surprise for many scientists. Generally, more or less it was assumed that due to Coulombs interaction the term of isobaric in the nuclei was a meaningless concept. This is the reason that the discovery of isobars was delayed. Originally isobars were called as the isobares and later this name was modified as isobars.
The term isobar was first used in 1918 Alfred Walter Steward. Isobar is derived from the Greek word iso means the same or equal and bars mean weight. The condition that mass number is the same implies neither the equal masses of atoms of corresponding nuclides nor the same mass of the nuclei. The phenomenon of isobars happens because there are a different number of neutrons in the atomic nucleus which compensates for the difference in the atomic numbers. In short, the atomic number is greater than the mass number.
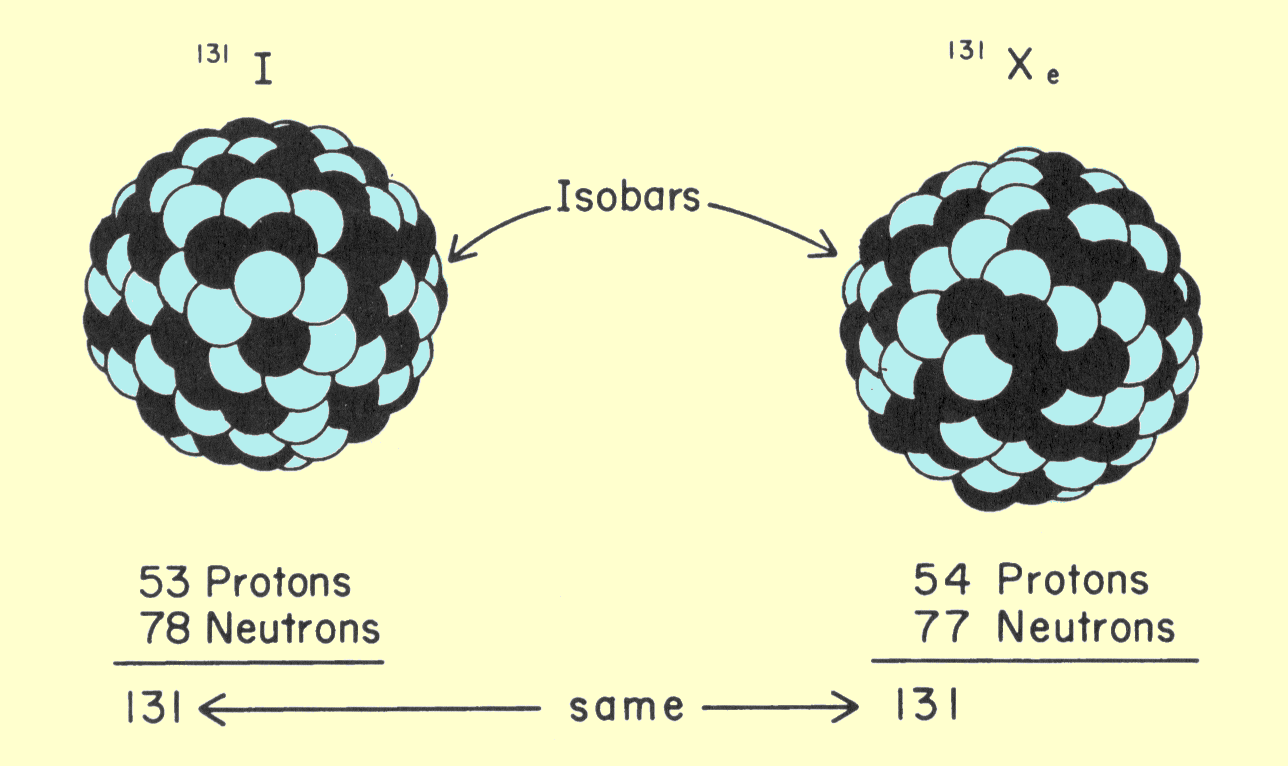
The isobars are atomic species that have the same mass number but their atomic number is different. Their chemical properties are different. However, physical properties are the same as they are related to the mass. In other words, isobars are also elements having the same number of nucleons i.e. sum of neutrons and protons. The series of elements having 40 mass number is a good example of isobars such as S, Cl, Ar, K, and Ca. In these elements, the number of particles is the same in the nucleus but they have a varying number of neutrons and protons. Thus, the evidence is supported that isobars have a different atomic number.
In the periodic table, isobars occur at different places. For example, ₃₂⁷⁶Ge and ₃₄⁷⁶Se are isobars. In the first nucleus, there are 44 neutrons and 32 protons. In the second nucleus, there are 42 neutrons and 32 protons and each nucleus contain 76 nucleons. As the number of protons and neutrons is having the same weight so an isobar can have fewer neurons and more protons and their total weight in the Isobar remains the same. Carbon has atomic number 6 and its atomic weight is 12. The isotope of carbon C14 has the same number of protons that are 6 but two extra neutrons are present and its atomic weight is 14. So, nitrogen and carbon 14 are having the same atomic weight and therefore, they are the isobars. Another example is the atomic number of calcium is 20 and the atomic number of argon is 18. Both of them have a different number of protons, but they have a mass number 40. Furthermore, there are a series of elements that are having the same atomic masses such as Cobalt, Nickle, Iron, and Copper. The atomic mass of these is 64 and but there is variation in their atomic number.
Many elements may have more than 2 isotopes but they are stable for only a short period. More than two, three, or four elements may be part of the same isobar. It may be for a much shorter time, but this phenomenon may happen.