Oxidation and Reduction (Redox) reactions and basically used for transforming matter from one form to another. These reactions find extensive use in pharmaceutical, biological, metallurgical and agricultural and many more areas. From burning of different types of fuels to obtaining energy for domestic, to the formation of rust, everything falls within the purview of redox processes. Some redox reactions are fast while others are slow. This shows the importance of these reactions.
Oxidation
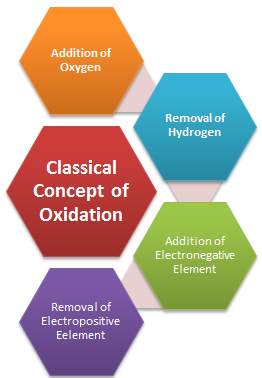
Initially, oxidation was used to describe the addition of oxygen to an element or a compound. The following reaction represents oxidation processes according to the limited definition of oxidation:
CH4 (g) + 2O2 (g) —— 2H2O (l) + CO2 (g)
After carefully examining the above reaction in which hydrogen has been replaced by oxygen prompted chemists to reinterpret oxidation in terms of removal of hydrogen from it and, because of this the scope of term oxidation was broadened to include the removal of hydrogen from a substance. As knowledge of chemists grew, it was natural to extend the term oxidation for reactions, which do not involve oxygen but other electronegative elements. The oxidation of magnesium with chlorine and sulphur etc. occurs according to the following reactions:
Mg (s) + Cl2 (g) ———- MgCl2 (s)
Mg (s) + S (s) ———— MgS (s)
These reactions encouraged chemists to consider not only the removal of hydrogen as oxidation, but also the removal of electropositive elements as oxidation. Thus the reaction:
2K4 [Fe (CN) 6] (aq) + H2O2 (aq) —————- 2K3 [Fe (CN) 6] (aq) + 2 KOH (aq)
This reaction is interpreted as oxidation due to the removal of electropositive element potassium from potassium Ferro cyanide before it changes to potassium ferricyanide.
Taking all the above equations into consideration we can define Oxidation in wider terms as the addition of oxygen/ electronegative element to a substance or removal of hydrogen/electropositive element from a substance.
Reduction
Similarly initially reduction was considered as removal of oxygen from a compound. However, the term reduction has been broadened in the same way as done for oxidation. Reduction includes removal of oxygen/electronegative element from a sub stance or addition of hydrogen/ electropositive element to a substance.
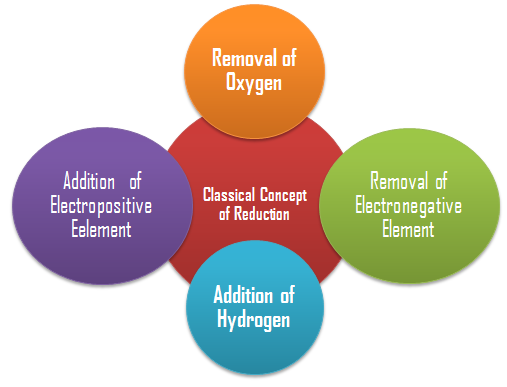
According to the definition given above, the following are the examples of reduction processes:
2 HgO (s) ——— 2 Hg (l) + O2 (g) (removal of oxygen from mercuric oxide)
2 FeCl3 (aq) + H2 (g) —— 2 FeCl2 (aq) + 2 HCl (aq)
(Removal of electronegative chlorine from Ferric Chloride)
CH2 = CH2 (g) + H2 (g) ——– H3C – CH3 (g) (Addition of Hydrogen)
2HgCl2 (aq) + SnCl2 (aq) ——- Hg2Cl2 (s) +SnCl4 (aq) (Addition of mercury to mercuric chloride)
In the above reaction simultaneous oxidation of chloride is also taking place because of the addition of electronegative element chlorine to it. Hence it was soon realised that oxidation and reduction always occur simultaneously. Therefore the word “redox” was coined for this class of chemical reactions.