Gases could be relatively difficult to transfer. For such purposes their conversion to the liquid state is quite essential. Getting through the liquefaction of gases will also let you know much more about the characteristics of the state of matter.
What is Liquefaction?
Liquefaction is the process under which an element is converted from its gaseous state to liquid state; by increasing pressure and decreasing temperature. Adjusting pressure and temperature results in the molecules coming closer which gradually turns them into a liquid.
Along with the change in temperature and pressure there are other physical factors that change including volume. The first person to set off with the in-depth study of conversion of gases to liquids in Carbon Dioxide was Thomas Andrew. Later, it was realized that most of the gases could be turned into liquids with appropriate change in the considerable physical factors including temperature, pressure and volume.
All thorough his experiments, Andrew concluded that only high pressure was not enough to convert gases to liquids. Also, that considerable change in temperature resulted in the gases deviating from their ideal behavior.
Critical Volume, Temperature and Pressure
Throughout his experiments, Andrew realized that above a certain temperature, it was not possible to covert gases into liquids. Even the highest pressure, it was important to adjust the temperature also. The apt temperature at which a specific gas could be converted to its liquid state has been termed as the critical temperature. With the increase in temperature, the pressure needed to liquefy a specific gas also tends to increase. This is generally the highest temperature at which the gas is likely to exist in its liquid state.
The critical constants are said to accomplish essential roles in the liquefaction of gases. These include critical pressure, volume and temperature. Just like the critical temperature (Tc); the pressure required for turning the gas in to liquid is called critical pressure (Pc) and apt volume is called critical volume (Vc).
Gases to Liquids
- The critical temperature is probably the highest temperature at which liquefaction of gas would occur. This temperature is likely to signify the change in the force of attraction among the molecules. Higher the critical temperature, higher are the forces of attractions among the element’s molecules and easier the liquefaction.
- Cooling and compression, both are required for the liquefaction of gases.
Isotherm of Carbon Dioxide
Since CO2 was first considered by Andrew for liquefaction, we would consider it for further understanding of the topic. An isotherm is the graph that represents suitable behavior of the element under different temperatures.
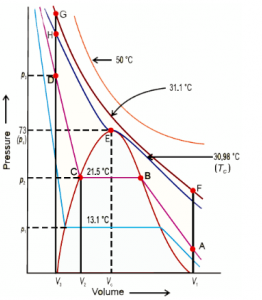
Studying the given isotherm will let you make out temperature wise change in the physical state of the element. In this change of physical state, pressure and volume tend to play the most vital role along with temperature. From the given graph, it could be noted that the gaseous state of Carbon Dioxide changes to liquid, at 30.98 degrees Celsius. At temperature lower than this, the curve tends to change while at higher temperature, there is no change in the curve. It is at the said temperature that the element deviates from its ideal gas behavior.