Avogadro’s law states that under the same temperature and pressure conditions, there’ll be equal volumes of different gases containing an equal number of molecules. This empirical relationship or known as the phenomenological relationship can be derived from a kinetic energy concept of gases under the ideal gas assumption. The law is almost valid for actual/real gases at nearly low P and high T.
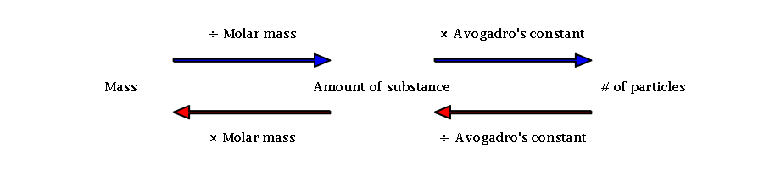
In one gram-mole of a substance, a specific number of molecules is defined as the MW (Molecular Weight) which is 6.022140857 × 1023 grams or 6.022 × 1023 grams, a number called Avogadro constant, also called Avogadro’s number.
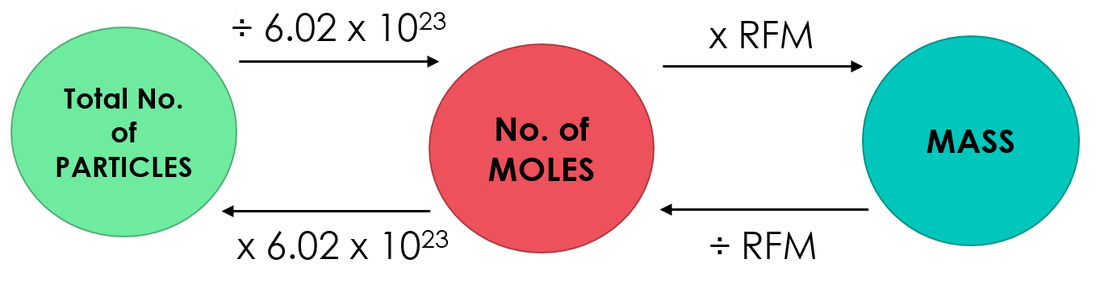
For instance, Oxygen’s molecular weight is 32.0, so that Oxygen’s one gram-mole contains a mass of 32 grams and has 6.022140857 × 1023 molecules.
One-gram of gas occupies a volume of about 22.4 litres at normal pressure and temperature (1 atm, 0 °C) and which is exactly the same for all gases, as per Avogadro’s law.
The now a day’s explanation of Avogadro’s law is “for a mass of an ideal gas particularly, the amount (number of moles) and volume of the gas are completely direct proportional, serve the temperature and pressure situations are constant.”
Avogadro’s law formula
The mathematical representation of Avogadro’s law is given by:
V ∝ n, or V/n = k
Where,
V = volume of the gas
n = amount of the gas (no. of moles of the gas)
K = constant for a given temperature and pressure
It can also be written as,
=V1/N1 = V2/N2
The formula of Avogadro’s law illustrates how the equal volumes of all gases consist of equal volumes of the whole of the gases consist of same no. of molecules with the same temperature and pressure situations. Putting it differently, it explains that two different gases having equal volumes will consist of the same number of molecules, considering that the pressure and the temperature are the same.
Moles into Grams
Another well-known measurement of a quantity is that moles can be converted into grams, by the formula:
Moles = grams/molar mass; M = Gms/M.M
So, to determine the substance’s molar mass, you have to use the modern periodic table. Simply, it’s calculated by totaling the individual atoms’ mass number. For example, if you’re calculating the molar mass of NaCl –
Mass no. of Na = 22.99 g/mol
Mass no. of Cl = 35.45 g/mol
Thus, molar mass (M.M) of NaCl = 22.99 + 35.45 = 58.44 g/mol
There are a lot of applications in physics and chemistry. For example, having a volume of 1 mol.of the gas at STP (Standard Temperature and Pressure) is 22.4L. So, the calculations are pretty hard.
Derivation from the ideal gas law
From Ideal gas law, Avogadro’s law’s derivation directly follows, that is:
Where,
R = gas constant,
T = Kelvin temperature,
P = pressure (in pascals)
Solving for V/n, we obtain
V/n = RT/P
On comparing,
K = RT/P
Where above is constant for fixed temperature and pressure.
Ideal gas law’s equivalent formulation can be described using Boltzmann constant kB, as
PV = NkBT,
Where,
N = number of particles in the gas, and also, the ratio of R over kB is equal to “Avogadro constant”.
V/N is a constant, where,
V/N = k’ = kBT/P
So, If P and T are considered at STP (standard conditions for temperature and pressure),
then k′ = 1/n0,
where n0 = Loschmidt constant.