Interestingly, you’ll be surprised to know that the discovery of Avogadro’s number was not actually done by Amedeo Avogadro. The value determination and mole concept of Avogadro’s number came into action after the death of Avogadro. Indeed, the Avogadro’s number is given its name in the honor of Avogadro’s work and his discovery.
The person who first calculated the total no. of particles exist in the substance was Josef Loschmidt, an Austrian teacher, who later was promoted as a professor at the Vienna University.
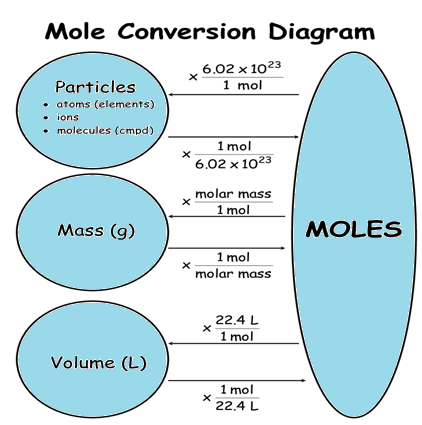
The number of units in Avogadro’s number’s one mole of any substance is called its molecular weight expressed in grams, which is equal to 6.022140857 × 1023. These units can be atoms, electrons, ions, or molecules, depends on the substance’s nature and character of the reaction taking place (if there’s any).
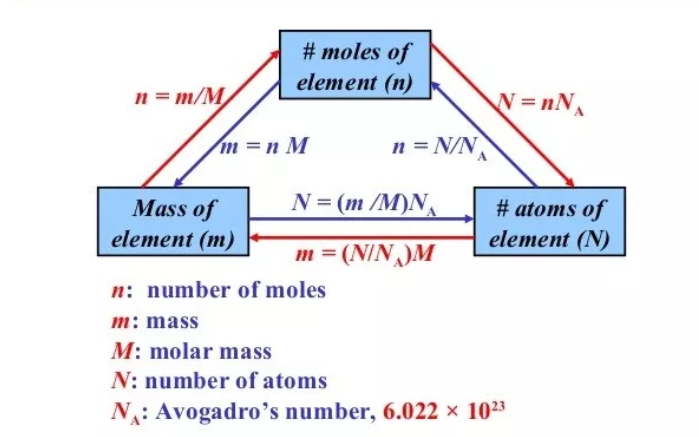
Mol-1 is the unit for Avogadro’s number, which means the measure of the substance is per mole. The Avogadro’s number is commonly represented by, “N”. Also, it’s expressed as NA.
Calculation of the number of molecules in 1 mole of a gas, the quantity is known as Avogadro’s number. The ‘number density of gas’ was close enough to 1.0 × 1019 molecules per cubic cm, and, it’s known that one mole of gas involves about 25 litres of volume i.e. (2.5 × 10 to the power 4 cubic cms) under regular conditions.
Making the use of these values, the approximate calculation of Avogadro’s number is (1.0 × 1019)(2.5 × 104) = 2.5 × 1023 molecules per mole.
It somewhat distracts from the accepted and approved value of 6.022 × 1023 molecules per mol., but magnitude’s order is definitely right.
According to the historical fact, Avogadro’s number’s value is as good as this approximate calculation was not acquired until 1865, when the Josef Loschmidt, an Austrian scientist in Vienna made a similar calculation to the exact one here but it was based on gas viscosity instead of on gas diffusion. In older times, often Avogadro’s number is known as Loschmidt’s number for this very reason.
Currently, generally, Loschmidt’s number is taken into consideration to mean a number of molecules of a gas in 1 cubic cm at 0-degree Celsius temperature and 1-atmosphere pressure (2.687 × 1019 molecules per cubic cm).
There are other methods by which Avogadro’s number and molecular sizes could be calculated, like from the surface tension or to spread surface oil film over water or the energy involved in the evaporation of a liquid.
6.02×1023 is known as the Avogadro’s number, in a mole, the number of representative particles. It’s the experimentally determined number. The smallest unit of a representative particle in which a substance normally exists. For most of the elements, the atom is the representative particle. Carbon, Helium, and Iron consist of carbon atoms, Helium atoms, and Iron atoms respectively. There are 7 elements which are diatomic molecules including H2, O2, Cl2, F2, I2, and Br2.
The molecule is their representative particle for these given elements. Similarly, all molecular compounds including H2O and CO2 are known to be the molecules and therefore their representative particle is molecule.
For the ionic compounds like Ca (NO3)2 and NaCl, formula unit is their representative particle. The mole of any substance consists of Avogadro’s number (6.02×1023) of representative particles.