It is quite certain that liquefaction of gases would be a star too far without considering the physical factors that include pressure, temperature and volume. The most appropriate factors for change in physical state of the element are termed as the critical factors.
What is Critical Temperature?
The critical temperature for a specific substance could be defined as the highest temperature at which that substance could exist in its liquid form. At any temperature above this, the element is likely to turn into the gaseous form. Other than the critical temperature, gases can be no longer liquefied despite the highest amount of pressure applied.
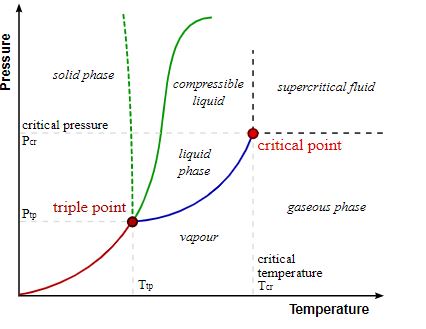
The given graph represents a triple point at which an element can prevail in all three states of matter and also the critical point at which the element liquefies. A glance at the graph would notify that pressure is plotted on the Y-axis while temperature is presented on the X-axis. The critical temperature would therefore be obtained from the x-axis and the y axis would provide with the required pressure for converting the element into liquid. Being the pressure required for liquefaction, it is known as the critical pressure.
A French physicist, Charles Cagniard de la Tour discovered the critical point of water in the year 1822. He was the one to observe that Carbon Dioxide could be liquefied at the 31 degree Celsius when 73 atm of pressure is applied. However, at a temperature higher than this, it could not be turned into a liquid even if 3000 atm of pressure was applied to it. The maximum temperature at which substances could be liquefied was termed as the Critical Temperature.
Important points to consider
- The critical temperature for water is observed to be 647K.
- The critical temperature for steel is 1533.15 K.
- It is important to note that critical point and boiling point are known to be two different concepts. While boiling point is the temperature at which the element could be converted to vapor; the critical point is achieved mutually with pressure and temperature. This is when the element is both in the liquid and gaseous state.
The critical temperature and the critical pressure of an element are major for defining its critical point. Beyond this, the substance is likely to take the form of a supercritical liquid. Regardless of the pressure applied to it, a substance cannot be converted into its liquid state at a temperature above the critical temperature. When the temperature is higher than the critical temperature, the molecules possess enough kinetic energy to overcome the intermolecular forces of attraction. The minimum pressure that is required to convert a gas to its liquid state is termed as the critical pressure. The combination of appropriate pressure and temperature for liquefaction is called the critical point.
Above the critical temperature, the substance exists in its supercritical fluid state. This is when the substance could fill a container completely like gas, but possesses the density that is equal to a liquid.