Chemical Thermodynamics: First law of thermodynamics
The first law of thermodynamics is explained as “The total change in internal energy of a system is the sum of the heat added to it and the work done on it.”The conservation of energy take place which essentially means that “energy cannot be created or destroyed” or that “the energy of the universe is constant”.The transformations of energy take place around us round the clock. For example, light bulbs transform electrical energy to light energy, and gas stoves change chemical energy from natural gas into heat energy. Similarly, plants perform one of the most biologically useful energy exchange on Earth: they convert the sun rays into the chemical energy stored within organic molecules.
A way of expressing the change in energy in a system, ∆U, is the sum of the heat added to the system, ∆Q, and the work done on the system, ∆W.
∆U = ∆Q + ∆W
Heat and work are designated as positive when they add energy to the system. This means that positive heat Q and positive work W adds energy to the system. We can conclude that the first law elaborates that you can increase the internal energy of a system by heating , or doing work on the system.However, this analogy does not provide the information of direction of process and does not determine the final equilibrium state of the reaction.
For an isolated system, ∆Q = ∆W = 0 and U = constant. Even though Q and W depend on the path, ΔU = Q – W does not depend on it. The change in internal energy of a system during any thermodynamic process depends only on the initial and final states, not on the path leading from one to the other. i.e. U is an intrinsic property of the system.
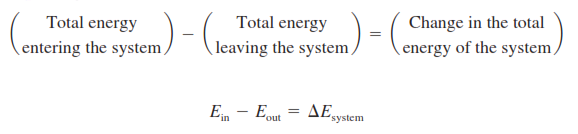
Figure : Energy balance for a closed system.
Where Total energy, E of a system is equal to the sum of its internal energy, U, kinetic energy, KE, and potential energy, PE.
Recall that W is equal to the negative external pressure on the system multiplied by the change in volume: W=−pΔV
where p is the out bond pressure on the system, and ΔV is the change in volume. This is specifically called “pressure-volume” work which is significant for gases.
For an isolated system, ∆q=0, so, ∆U = – ∆w
For system involving mechanical work only, ΔU = q – pdV
At constant volume i.e. isochoric process, ΔU = qv
For Isothermal Process, ΔU = 0 or q = – pdV =-W
For adiabatic process, Δq = 0 or ΔU = W
At constant pressure, ΔU = q – pdV
Where,
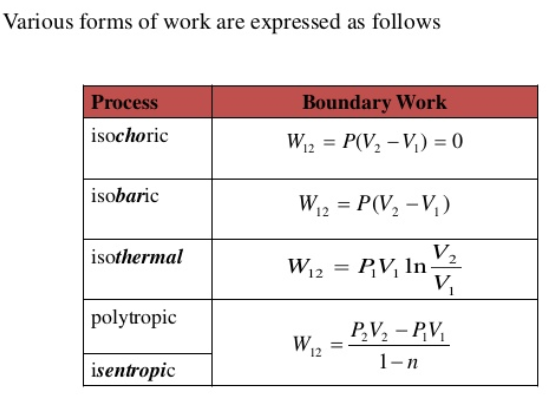
Figure : Different forms of work
One important thing to keep in mind about the First Law is that the transformation of energy is not 100% efficient. In our light bulb example, you can transform electrical energy into a usable form of light energy, but in the process, you create unusable energy in the form of heat.