Chemical Thermodynamics: Measurement of Internal energy change, δU and enthalpy, ΔH
Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount Calorimeter is a device for measuring the heat produced during an electrical, mechanical or chemical reaction, and for calculating the heat capacity of various materials.
The heat capacity of most systems is not a constant value. It depends on the state variables of the thermodynamic system being analyzed. Heat capacity is dependent on temperature, as well as on the pressure and the volume of the system and their path.
Chemical reactions, such as combustion, do not take place in a solution. In order to efficiently measure the heat produced by combustion reactions, chemists use a bomb calorimeter. A bomb calorimeter is mostly used for the measurement of internal energy change. In this technique, a steel vessel called bomb is immersed in a water bath to ensure that no heat is lost to the surrounding. A combustible substance is then burnt in oxygen gas supplied into the bomb. The heat evolved is absorbed by the water around the bomb and the change in temperature is measured. Energy changes associated with the reaction are measured at constant volume since the volume does not change in a fully sealed bomb calorimeter. Since the volume remains constant, work done by the system is 0. Which means that internal energy is associated with heat only.
Internal energy change in this process is calculated as:
ΔU=qv= mcvΔT
Where,
m= mass of water
cv= specific heat capacity at constant volume
ΔT = temperature difference
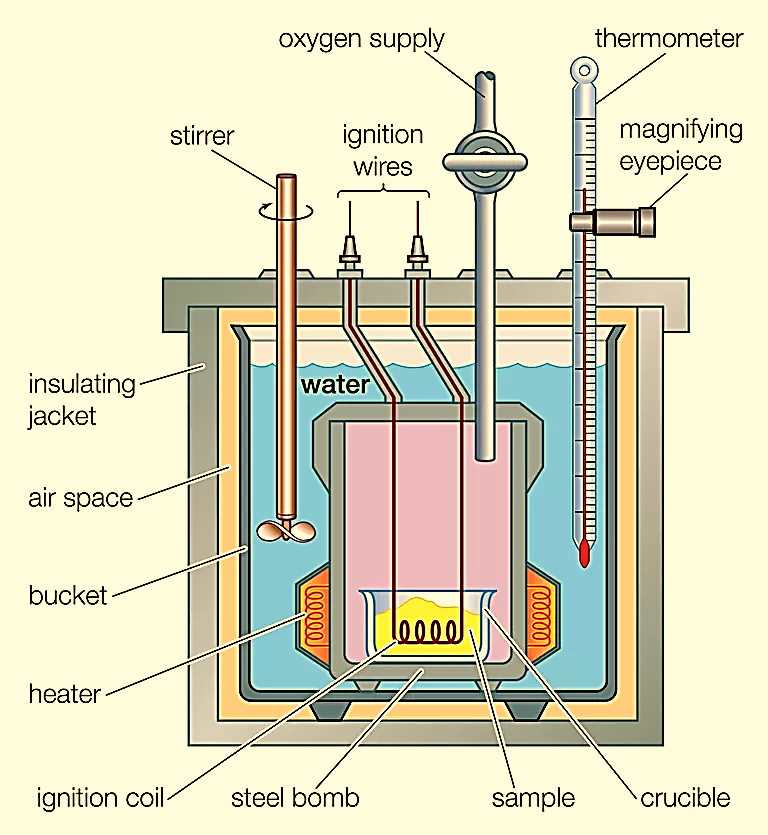
Figure 1: Bomb calorimeter.
When the reaction occurs, the change in temperature will be proportional to the enthalpy released by the reaction. A Coffee-cup calorimeter is used to calculate the enthalpy change due to its ease of its procedure. Here, the cup is partially filled with a known volume of water and a thermometer is inserted through the lid of the cup such that its bulb is below the water surface. In a chemical reaction, the heat of reaction is absorbed by the water. The water temperature change is measured to calculate the amount of heat that has been absorbed or evolved. The cup is made of polystyrene foam which is a very good insulator, very little heat energy escapes the system.
The enthalpy change in this process is calculated as:
ΔH=qp= mcpΔT
Where,
m= mass of water
cp= specific heat capacity of water at constant pressure
ΔT = temperature difference
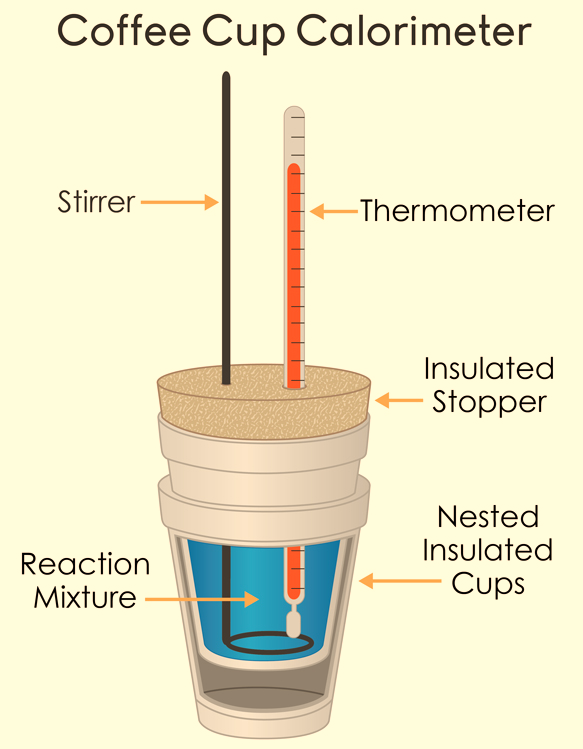
Figure 2: Coffee Cup calorimeter
| Coffee cup calorimeter | Bomb calorimeter |
| Mainly used for solution-based chemistry with negligible volume change. | Used for solid or gaseous substances |
| Change in the water temperature is used to calculate the heat absorbed in the reaction. | The reaction takes place in a sealed metal container which is the bomb vessel which is a sealed high-pressure vessel. When a sample is fired, the heat of the reaction is totally absorbed into the bomb vessel walls to measure the temperature change. |
| Often used by University students for learning | Good for industrial used also. The bomb vessel can be reused againwith a simple cooling cycles |
| Less accurate due to heat loss to the environment and human error in reading the temperature change. | More accurate due to low human error. |