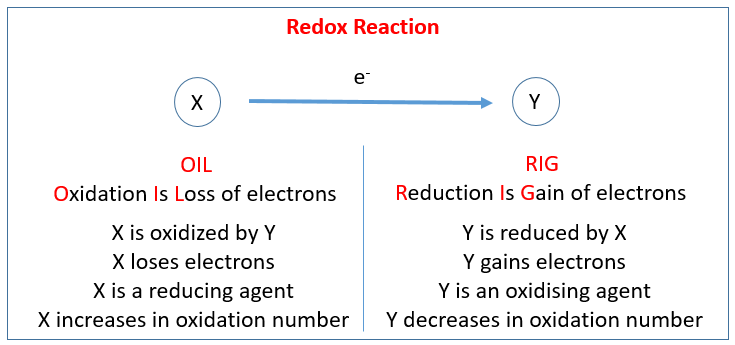
The reactions in which the oxidation states of atoms are changed are known as Redox reactions. They are characterized by transfer of electrons. The chemical species in which the electron is added is said to have been reduced, while the chemical species from which the electron is subtracted is said to have been oxidized.
Types of Redox Reactions
1) Combination reactions
It may be denoted in the following manner:
A + B —> C
Both A and B or either of them must be in the elemental form for such a reaction to be a redox reaction. All combustion reactions, which make use of elemental di-oxygen, as well as other reactions involving elements other than di-oxygen, are redox reactions. Some important examples of this category are:
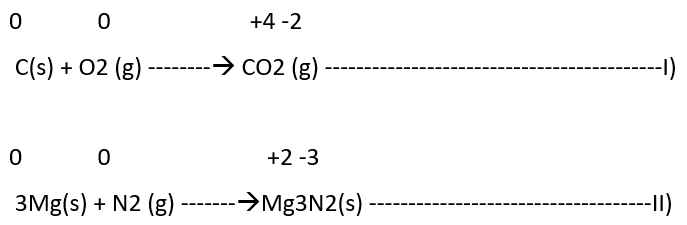
2) Decomposition reactions
Decomposition reactions are the opposite of combination reactions. It leads to the disintegration of a compound into two or more components. At least one of this must be in the elemental state. Examples of this class of reactions are:

It must be noted here that all decomposition reactions are not redox reactions. For example, calcium carbonate decomposition is not a redox reaction.
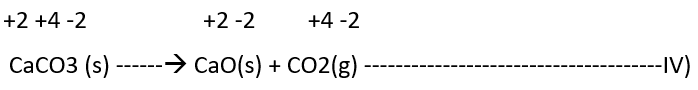
3) Displacement reactions
In a displacement reaction, an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element. It may be denoted as:
X + YZ —-> XZ + Y
Displacement reactions are categorised into two types: metal displacement and non-metal displacement.
a) Metal displacement: A metal in a compound can be displaced by another metal if not in combined state. A few such examples are:
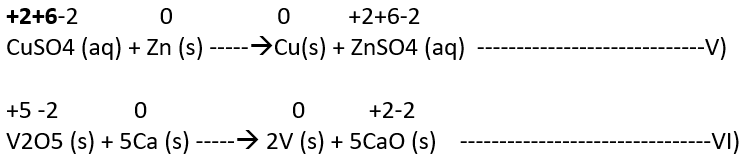
In each case, the reducing metal has more capability to lose electrons as compared to the one that is reduced which makes a reducing metal a better reducing agent.
b) Non-metal displacement: The non-metal displacement redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement.
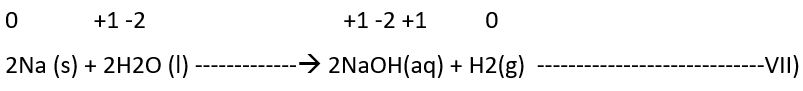
4)Disproportionation reactions
These are a special type of redox reactions. In this reaction an element in one oxidation state is simultaneously oxidised and reduced. The decomposition of hydrogen peroxide is an example of the reaction. In this the oxygen experiences disproportionation.
![]()
Here the oxygen of peroxide, which is present in –1 state, is converted to zero oxidation state in O2 and decreases to oxidation state of –2 in H2O.