Oxidation-reduction reactions have many diverse applications ranging from industries to our daily lives. Some of the important applications of Redox Reaction are as follows:
Redox Reaction in Electrochemistry
The batteries which are used for generating DC current use redox reaction to produce electrical energy.
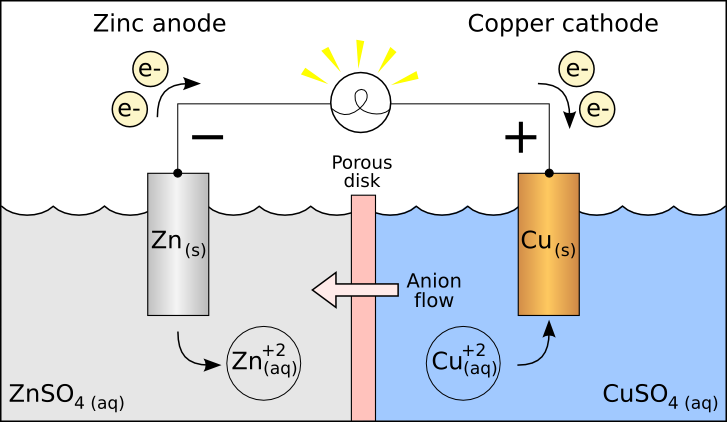
Batteries also called as electrochemical cells used in our day-to-day life are also based on redox reactions. For example, storage cells which are used in vehicles to supply all the electrical needs of the vehicles.
Redox Reaction in Combustion
Combustion involves oxidation-reduction reaction and hence it is a redox reaction. An explosion is a swift form of combustion and hence explosion can be treated as a redox reaction. Even the combustion in space shuttle works on redox reactions. The combination of powdered aluminium and ammonium perchlorate inside the rocket boosters gives rise to oxidation-reduction reaction.
Redox reaction in Photosynthesis
Water and carbon dioxide are converted by plants into carbohydrates and this process is defined as photosynthesis. The reaction is given below:
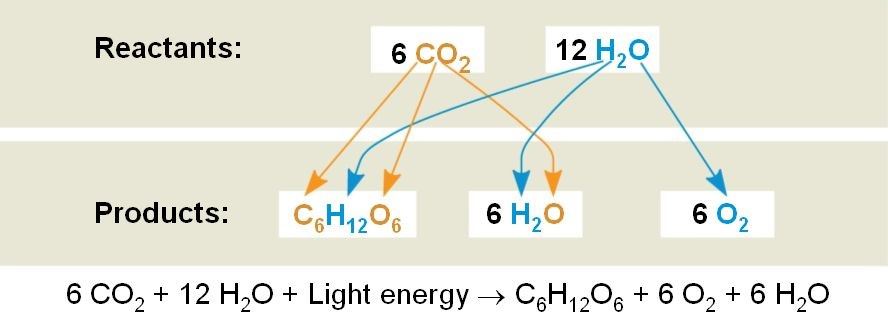
From the reaction above, we can see that the carbon dioxide is reduced to carbohydrates while the water gets oxidized to oxygen and hence it is a redox reaction. Sunlight provides energy for this reaction. This reaction is a source of food for animals and plants.
Extraction of metals
Metal oxides can be reduced to metals by using a suitable reducing agent. For example, ferrous oxide is reduced to iron in the blast furnace using coke as the reducing agent.
Production of chemicals
Many chemicals which we use in our daily lives such as caustic soda, chlorine, fluorine, etc., are produced by electrolysis which is based on redox reactions.
Quantitative analysis
Redox reactions are very useful in quantitative analysis by redox titrations. These titrations involve the reactions between oxidising and reducing agents and help in estimating the amount of unknown substances in solutions. These reactions are useful in pharmaceutical industries.
Real Life Uses of Redox Reaction
- Electrolysis is used in the production of some important chemicals which in turn is based on redox reactions. Many chemicals like caustic soda, chlorine, etc. are produced using redox reactions.
- Oxidation-Reduction reactions also find their application in sanitizing water and bleaching materials.
- The surfaces of many metals can be protected from corrosion by connecting them to sacrificial anodes which undergoes corrosion instead. A common example of this technique is the galvanization of steel.
- Oxidation processis used in the industrial production of cleaning products.
- Nitric acid which is a component in many fertilizers is produced from the oxidation reaction of ammonia.
- Redox reactions are also used in in the process of electroplating by applying a thin coating of a material on an object. It is used in the production of gold-plated jewellery.
- It is used for separatingmetals from their ores. One such example is the smelting of metal sulphides in the presence of reducing agents.
Oxygenis the main source of oxidation and therefore redox reaction or oxidation-reduction reactions are responsible for food spoilage.