Calcium carbonate, one of the popular chemicals is encountered by us first in the classrooms in the form of chalk (form of CaCO3). It occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc. It is a white insoluble powder-like substance which is found in earth’s crust. It is prepared by passing carbon dioxide gas through calcium hydroxide which is commonly known as slaked lime.
Ca (OH)2 + CO2→ CaCO3 + H2O
When it reacts with a dilute acid, it gives carbon dioxide as a by-product.
CaCO3+H2SO4 → CaSO4+H2O+CO2
At 1200K, calcium carbonate decomposes and liberates carbon dioxide along with calcium oxide.
CaCO3 → CaO + CO2
Calcium carbonate is used extensively in many industrial processes. Some of them are mentioned below:
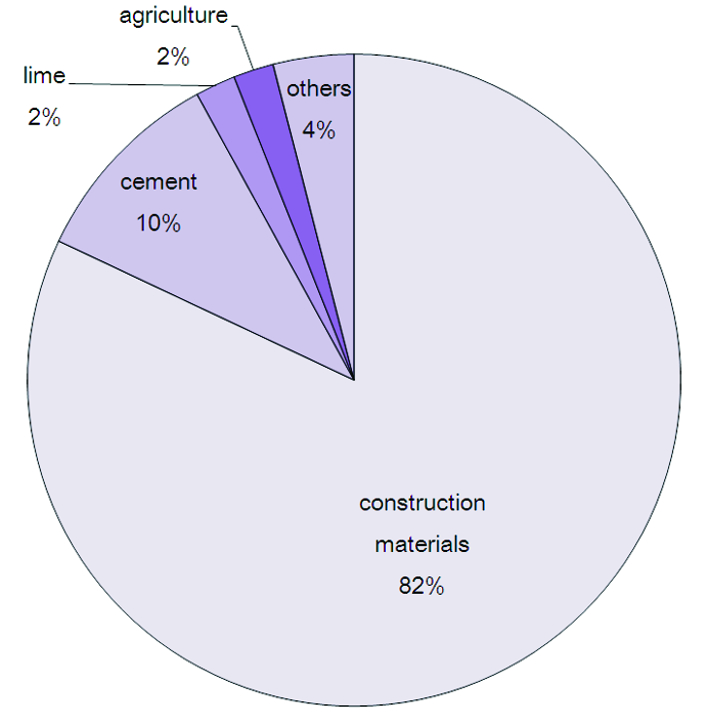
Construction Industry
Mostly calcium carbonate is used either as a building material, or limestone aggregate for road building It is also used as an ingredient of cement. However, because of weathering mainly caused by acid rain,calcium carbonate can no longer used for building purposes on its own. It can only be used as a raw primary substance for building materials.
Purification of iron
Calcium Carbonate is used for purifying iron from iron ore in a blast furnace. It is processed in situ to give calcium oxide. This forms a slag with various impurities present, and separates from the purified iron.
Oil industry
Calcium carbonate is added to drilling fluids to act as formation-bridging and filter cake-sealing agent. Along with this it is also a weighting material which increases the density of drilling fluids to control the downward pressure.
pH corrector
Calcium carbonate is added to swimming pools, as a pH corrector for maintaining alkalinity and offsetting the acidic properties of the disinfectant agent.
Sugar Industry
It is used as a raw material in the refining of sugar from sugar beet; it is processed in a kiln with anthracite to produce calcium oxide and carbon dioxide. This burnt lime is then slaked in fresh water to produce a calcium hydroxide suspension for the precipitation of impurities in raw juice during carbonization.
In latex gloves
For growing Seacrete, calcium carbonate is a main source. It is a common filler material for latex gloves with the aim of achieving maximum saving in material and production costs.
Paper Industry
Ground calcium carbonate (GCC) and PCC are used as filler in paper because they are cheaper than wood fibre. GCC are the most important types of fillers currently used in terms of market volume.10–20
Extender in Paints
Here typically 30
Ceramic glaze applications
Calcium carbonate is known as whiting, and is a common ingredient for many glazes in its white powdered form.