Typically, the atomic radius is measured as half of the distance between the nuclei of the two bonded atoms. Groups in the periodic table are just like the vertical columns and by moving down the group the principal quantum number is increased by one. It means that energy levels are filled by the electrons and farther away from the center of atom the nucleus. By increasing the number of protons, the number of electrons is also increased. Now, more room is required for electrons to move around and many electrons can be hold by each energy level. For example, hydrogen is first element in group I elements and it has only one electron. But lithium has three electrons and its size is larger. Trend for atomic and ionic radii moving down the group is almost same.
Trends in the Atomic Radii
By moving down in the group, the number of occupied energy levels are increased from 2 to 6 and radium of an atom of the element is increased from 134 pm to 225 pm. Atomic radius increases from top to bottom of the elements and an additional electron shell or energy level are being added to each successive element. The increase in atomic radii is due to the electron shielding but the situation is quite complex as the principal quantum number n is not constant. It may seem reasonable to say that it is due to the successive addition of the electrons. However, it is a dramatical fact that radius is dependent on the nuclear charge.
Irregularities in the atomic radii of both group I and group II elements can be explained by the variations in the effective nuclear charge which is experienced by the electrons in the outer most orbitals of the elements. If the effective nuclear charge is greater the outer most electrons are more strongly attracted by the nucleus and atomic radii become smaller.
Trends in the Ionic Radii
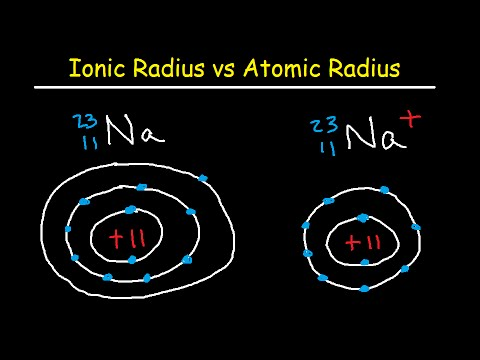
The size of the ions is not the same as their parent atom. The size of a positive ion or cation is smaller than the original atom as they have lost their electron or electrons. While the size of negative ions or anions is greater than the parent atoms as they have gained the electrons.
Ionic radii also follow the same vertical trend as the atomic radii. Such as for the ions with the same charge the ionic radius increases down the column. The reason is also the same as it is for atomic radii that are shielding by the filled inner shells that produce the little change in the effective nuclear charge that is felt by the outer most electrons. The principal shells with the larger values of n are lying at the successively greater distances from the nucleus.
When one or more electrons are removed from the neutral atoms, then the repulsion between the electrons in the same principal shells is decreased because of the presence of the few electrons and the effective nuclear charge felt by the remaining electrons is increased because there are only a few electrons for shielding.