Sodium hydroxide is also known as caustic soda. Its chemical formula is NaOH.
Preparation of sodium hydroxide:
Sodium hydroxide is manufactured by the electrolysis of a saturated solution of sodium chloride in the following type of cells.
Mercury cathode cell or Castner Kellner cell: this cell consists of a rectangular iron tank in which mercury flows along the bottom of the cell and is made cathode. The brine solution flows in the same direction and anode is made up of the number of graphite blocks. When the electric current pass through the cell, the following reactions takes place at both electrodes: $NaCl \to Na^{+}+ Cl^{-}$
At anode: $2Cl^{-} \to Cl_{2}+ 2e^{-}$
At cathode: $Na^{+}+ e^{-} \to Na$
Na + Hg NaHg (sodium amalgam)
Since in this cell cathode is mercury, the sodium atom produced dissolve in mercury and form an amalgam. The sodium amalgam flows out and is reacted with water to give sodium hydroxide and hydrogen gas.
$2NaHg+2H_{2}O \to 2NaOH+2Hg+ H_{2}$
The clean mercury is recycled back to the electrolysis tank.
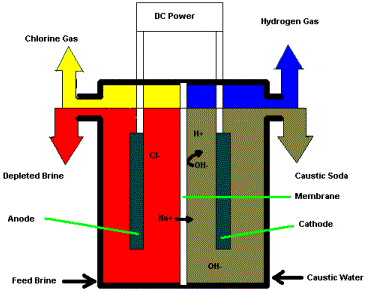
Diaphragm cell: In Castner Kellner cell there is one problem which is the chlorine gas evolved at anode tends to dissolve and react with sodium hydroxide forming sodium hypochlorite and hydrogen gas is evolved. If hydrogen and chlorine gases mix and react, the reaction can be explosive. In the presence of sunlight, a photolytic reaction may take place which produces chlorine atoms leading to an explosive chain reaction with hydrogen. Hence, it is necessary to prevent the contact of chlorine with the solution. For this, an asbestos diaphragm is used which separates the anode and cathode. This reduces the chances of chlorine produced at the anode can mix and react with NaOH produced at cathode. Thus, it reduces the chances of side reaction of chlorine forming hypochlorite. When the current is passed through carbon which is anode and wire gauze the cathode, electrolysis begins. Chlorine gas produced at carbon made anode and sodium hydroxide is formed at the cathode.
$2NaCl_{(aq)}+ 2H_{2}O_{(l)} \to 2NaOH_{(aq)}+ H_{2(g)}+ Cl_{2(g)}$
Properties of the sodium hydroxide:
- Sodium hydroxide is a white crystalline deliquescent solid. It’s melting point is 951 K.
- Sodium hydroxide is readily soluble in water to give an alkaline solution. It also neutralizes acids forming salt and water as shown here:$NaOH+HCl \to NaCl+ H_{2}O$
- The crystals of sodium hydroxide are deliquescent. They absorb moisture from the air. On prolong exposure sodium hydroxide can absorb carbon dioxide forming a white crust of sodium carbonate at the surface as shown here: $2NaOH+CO_{2} \to Na_{2}CO_{3}+ H_{2}O$
- Sodium hydroxide’s aqueous solution is soapy and has a strong corrosive reaction on our skin.
- An aqueous solution of sodium hydroxide contains a large number of hydroxyl ions and precipitates insoluble metal hydroxides from solutions containing metallic ions. The hydroxides of zinc, aluminum, lead dissolves in excess of sodium hydroxide giving clear solutions of respective soluble complexes. These soluble complexes can also be obtained from sodium hydroxide’s reaction with the metals. The reactions are given below:
$AlCl_{3}+ 3NaOH \to Al(OH)_{3}+ 3NaCl $
$Al(OH)_{3}+ 3OH^{- } \to [Al(OH)_{6}]^{3-}$