Chemical Thermodynamics: Phase transition
For any phase transition, enthalpy change can be experimentally quantified and tabulated. Each of the phase transitions has an associated latent heat which is required for the transformation.
The process that turns solid to liquid is referred to as fusion. The enthalpy of this change is calculated as:
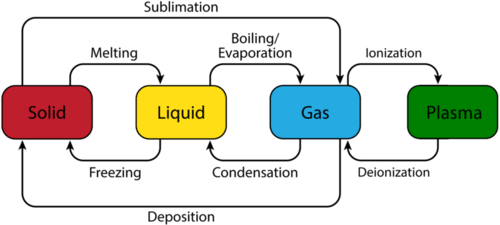
Figure :Heating curve diagram
In the figure above, a solid substance gains kinetic energy resulting in temperature change. For the calculation of heat required for temperature change, specific heat (C) of solid phase is used. At the melting point, latent heat is absorbed to break intermolecular forces and increase kinetic energy to form liquid wherein the temperature remains constant. For the calculation of heat required for phase change, molar heat of fusion is used.The liquid then gains K.E to increase its temperature until its boiling point where specific heat of liquid is used for calculations. Liquid –> gas change requires latent heat for breaking the intermolecular forces between particles to form gas. Here molar heat of vaporization is required for calculations.
| Parameter | Phase Transition | Enthalpy |
| molar heat of fusion (melting) | solid –> liquid | +6.0 kJ/mol |
| molar heat of vaporization | liquid –> gas | +40.7 kJ/mol |
| specific heat of ice | Ice –> Ice | 2.09 J/g•°C |
| specific heat of the water | Water –> Water | 4.18 J/g•°C |
| specific heat of steam | Steam –> Steam | 1.84 J/g•°C |
Figure : Parameters used for transition of water from one state to another.