The electronic configuration of an atom is an illustration of the layout of the electrons which are distributed among the orbitals and subshells. By using the idea of the configuration of the electrons it is possible to understand the various chemical and physical properties of the elements. The chemistry and behavior of the elements can be determined by studying the number of valance electrons in the outermost shells. Many principals are designed on the scientific bases to study the electronic configuration. Electronic configuration has much significance as it helps to study various physical and chemical properties.
Electrons fill the orbitals of the atom in such a way that the energy of the atom is at the minimum level. So, electrons fill the energy levels as defined by Aufbau’s principle. Another rule is defined by the Pauli and he defined a set of the unique quantum numbers for each of the electron. The Pauli exclusion principle states that all four quantum numbers for any of the two electrons in an atom can never be the same. Hund’s rule states that the pairing of electrons in the orbital only takes place when there is one electron in each subshell.
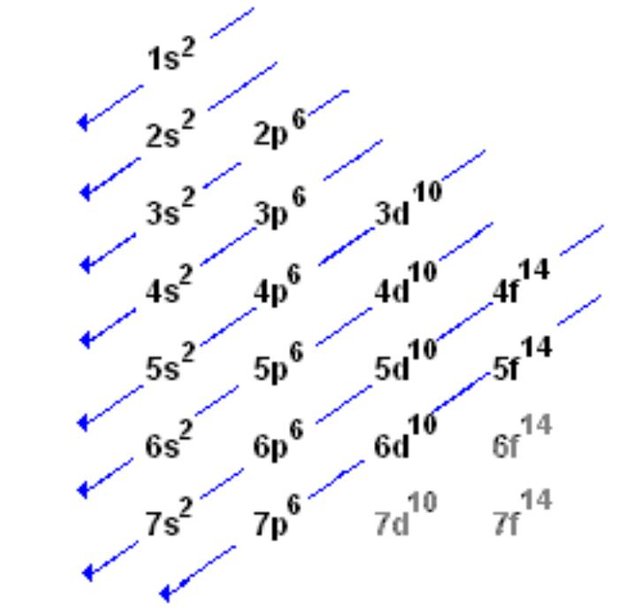
In the periodic table, elements are placed according to their atomic numbers that how many protons they have. In neutral atoms, the number of electrons is equal to the number of the protons and atomic number can be easily determined by using the electron number. Carbon group the group 14 elements have four electrons in the outer shell. Typically, the sharing of electrons is observed to complete the valance shells by forming the multiple bonds with the other atoms. Each column in the periodic table reflects the number of the electrons in the valance shells of each of the element which in turn strongly determine how the element will react. Orbitals and electrons are very closely related; however, orbitals provide a much accurate picture of the electronic configuration of an atom. It is due to the fact that orbitals specify the position and shape of the regions of the space that is occupied by the electrons.
The general electronic configuration of group 14 elements is ns2np2. There are two electrons in the outermost p orbitals of these elements. The electronic configuration for each element of this group is given below.
• Carbon [He]2s2 Sp2
• Silicon [Ne]3s2 3p2
• Germanium [Ar]3d10 4s2 4p2
• Tin [Kr]4d10 5s2 5p2
• Lead [Xe]4f14 5d10 6s2 6p2.
The electronic configuration of group 14 elements shows that these elements have four electrons in their valance (ultimate shell). Two of the electrons are in the s orbital and the remaining two are in the p orbital. So, they have an electronic configuration of s2p2 in their valance shell. The penultimate shell of carbon contains the s2 electrons, silicon has s2p6 electrons and germanium contains the s2p6d10 electrons and is unsaturated. This is the reason that carbon is different from the silicon and both of them are different from other elements of this group.