Chemical Thermodynamics: Standard enthalpy of dilution
When oneunit volume of liquid material is combined with a solvent liquid to achieve the desired concentration, it is referred to as dilution.
Addition of strong acid in water often generates heat. When a solvent like acid or base is transferred from its pure state or fully concentrated state to water and form a solution phase, enthalpy of dilution/hydration is the energy change involved in the dilution of solvent in solution at constant pressure.
Since dilution changes the ratio of solute to solvent, a ratio of concentration tovolume can be used for almost all calculations involving dilutions:
CiVi = CfVf
Where C is the concentration of the solute and V is the volume of the solution at initial (i) and final (f)conditions. The concentration of a solution is how much of the soluteis present per unit of volume. An easy way to see how this equation works is to use units of molarity and litres:
MiVi = MfVf
Molarity is used to calculate the molecules of a substance per unit volume. Specifically,molarity is the number of moles of a substance per litre of the solution. It is reported inmoles per litre or with a capital M for a molar.
(moles/liter)i x (liters)i = (moles/liter)f x (liters)f
Therefore,
molesi = molesf
Thus we see that regardless of the changes in volume, the total quantity of solute remains unchanged. Likewise, unknown quantitate can be easily calculated.
Ci = Cf [Vf/Vi]
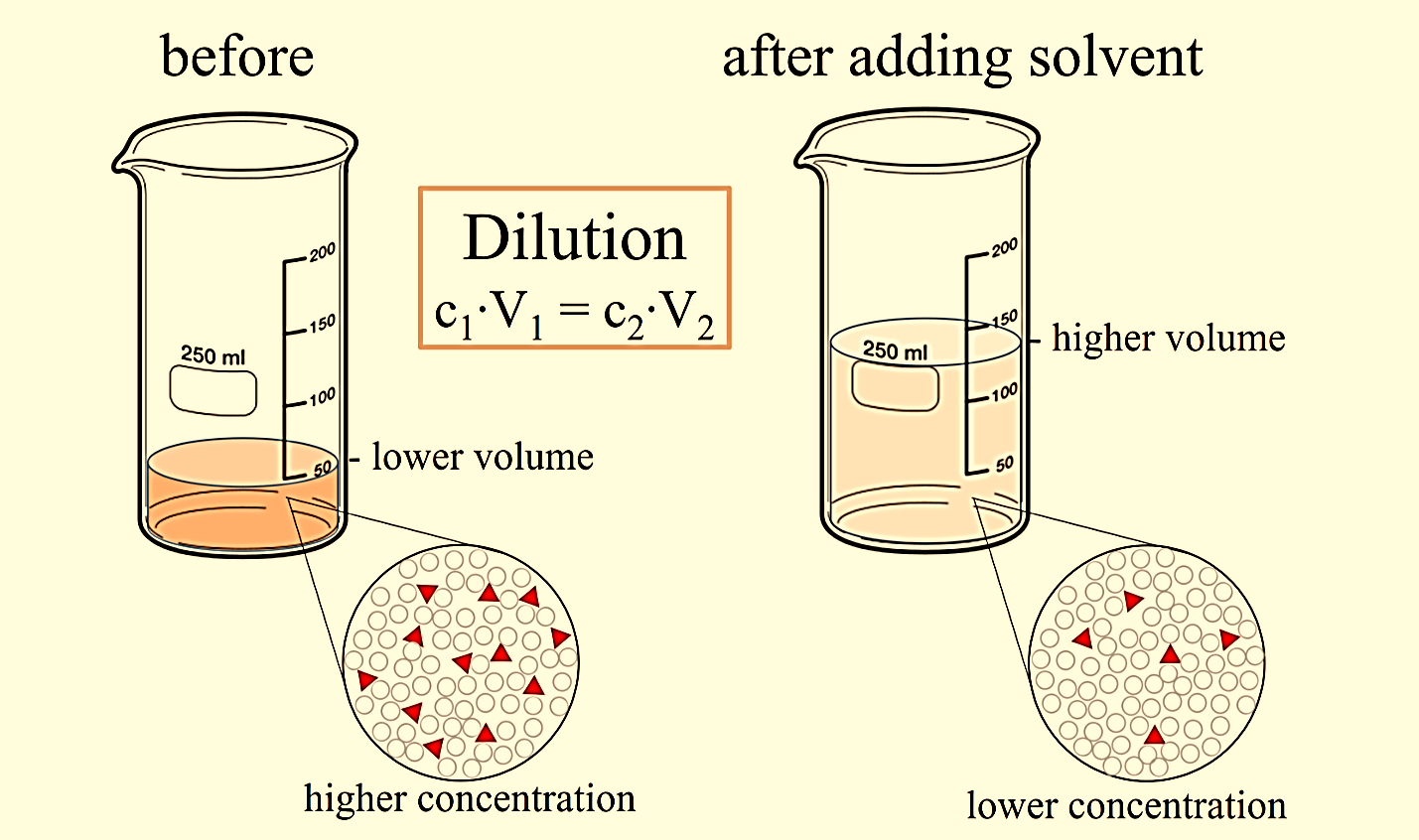
Figure 1: Dilution
Accordingly, when the pure liquid is mixed with water or any other base solution it can be considered as the dissolution process and in this case, the heat of dilution is the same as heat of the solution. The difference between dilution and dissolution is that in dilution the solute is in the liquid phase while in dissolution the solute can be in any phase. Another difference is that enthalpy of solution is dependant upon the amount of solvent added. A special case of enthalpy of dilution is at infinite dilution when a sufficiently large amount of solvent is added to the base so that further dilution does not produce any change in heat. This point is fixed and defined as the enthalpy of solution at infinite dilution.
For example,
Sulphuric acid has a very high affinity for water which means that the hydration of the acid molecules forms ions that are much lower in energy than the acid itself. Conc. Sulphuric acid is very hazardous and highly corrosive chemical. Hence, even though it is sold in concentrated form, in most chemical reactions a diluted version is used. When sulphuric acid mixes with water, the water molecules are protonated very quickly:
H2SO4 + H2O → HSO4- + H3O+; K1>>1
HSO4-↔ SO42- + H+; K2 0.012 (298 K)
In the first rapid reaction, the formation of stable HSO4- ions results in the loss of energy dissipated as heat. Hence, the dilution must always follow the order of acid to water so that the equilibrium favours the protonation of water where acid is a limiting agent.