Chemical Thermodynamics: Thermodynamic equilibrium
A clear difference between thermodynamics and kinetics is that thermodynamics tells us whether the reaction will occur or not in order to predict the final state. And, kinetics tells us how fast the reaction will occur and reach equilibrium. A system in the state of minimum energy is at equilibrium. This energy can be measured as Gibbs free energy when the reaction occurs at constant temperature and pressure. It deals with the relationships and conversions between heat and other forms of energy.
Thermodynamic equilibrium is the state in which no spontaneous changes occur in a system. It is attained when three types of equilibrium states are achieved.
- Thermal equilibrium: ∆T = 0
- Mechanical equilibrium: ∆P = 0
- Chemical equilibrium follows Le Chatelier’s principle.
Chemical equilibrium applies to reversible reactions that follows the same thermodynamic path in both directions
Consider the following reaction:
![]()
Le Chatelier’s principle states that if a change is applied on a system at equilibrium, the position of the equilibrium will shift in a direction such that the change is opposed, and the equilibrium is maintained. At the beginning of the reaction, the rate that the reactants are changing into the products is higher than the rate that the products are changing into the reactants. When the net change of the products and reactants is zero the reaction reaches equilibrium.
To determine the quantity of each compound present at equilibrium, the equilibrium constant is an essential variable. The equilibrium constant, K is the relative concentration of products and reactants at equilibrium.
Consider the following generic equation:
aA + bB↔cC + dD
The upper-case letters are the molar concentrations of the reactants and products. The lower-case letters are the coefficients that balance the equation.
To determine the equilibrium constant (Kc also written Keq):
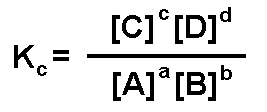
A reaction is favored when K > 1. K has no units and it is dimensionless. Likewise, the concentration of solutes should be expressed as moles per litre (M) and the concentrations of gases should be expressed in bars.
►Gases are measures using their Pgas, pressure instead of concentration
► 1 bar = 105 Pa; 1 atm = 1.01325 bar
Concentrations of pure solids, pure liquids and solvents are omitted because they are unity at the standard state.
Consider the following reaction:
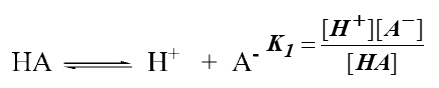
When the reaction is reversed, reciprocal of the equilibrium reaction is used:
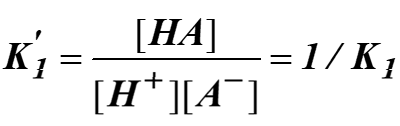
Thermodynamic constant, K depends on the chemical nature of the reactants and products. We measure ∆G by measuring the concentration of A, B, C and D once the reaction is in equilibrium.
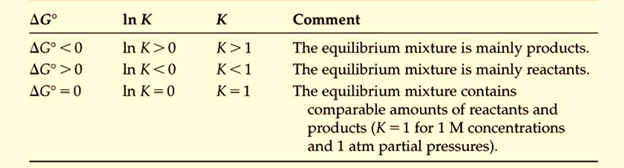
Figure 1: Relationship between the standard free energy change and the equilibrium constant for the reaction where ∆G = -RT ln K
The Arrhenius Equation relates the rate at which a reaction occurs to the temperature. In this case, activation energy must be overcome for a chemical reaction to occur.
k = A exp(-Ea/RT)
Or
log k = log A – (Ea/2.303RT)
Where A is an experimentally derived factor, Ea is the activation energy for the reaction, R is the ideal gas constant and T is a temperature in Kelvin.