Some Basic Principles and Technique
Organic Chemistry is a branch of chemistry which deals with the study of structures, properties and reactions of organic compounds. Here carbon containing chemical compound is known as organic compounds. Study of its structure helps determine its chemical composition and formula. This chapter talks about some basic concepts, structure and reactivity of organic compounds that are formed as a result of covalent bonding.
Tetravalency of Carbon
The atomic number of carbon is 6 and electronic configuration is 2, 4. This shows it has 4 valence electrons. Thus carbon is always tetra covalent and forms 4 covalent bonds with other atoms. Tetravalency of carbon gives it a tetrahedron shape.
Catenation:
Catenation is nothing but the self-linking property of carbon. Catenation is the main reason for existence of large number of carbon compounds.
Functional Groups
It is defined as an atom or a group of atoms present in a molecule which largely determines the chemical properties.
| CLASS OF ORGANIC COMPOUNDS | NAME OF FUNCTIONAL GROUP | STRUCTURE |
| Alkenes | double bond | =C=C= |
| Alkynes | triple bond | – C Ξ C – |
| Halogens | halogen | – X ( F,Cl,Br,I ) |
| Alcohols | hydroxyl | -OH |
| Aldehydes | Aldehydic(formyl) | -CHO |
| Carboxylic acids | carboxyl | -COOH |
| Acid amides
Primary amines |
amides
amino |
-CONH2
– NH2 |
Homologous series:
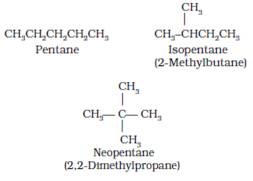
It is a family or group of organic compounds which are structurally similar. All members of it contain the same functional group and similar chemical properties. Any 2 adjacent members of it differ by –CH2 group. The phenomenon is called as homology and the individual members of this group are called homologues.
Isomerism
Two or more compounds which have same molecular formula but different physical and chemical properties are called isomers. And the related phenomenon is known as Isomerism.
Resonance Effect:
Due to the interaction of 2 pi bonds or between a pi bond and a lone pair of electrons present on an adjacent atom, polarity is produced in the molecule. This is known as Resonance Effect. It is divided into 2 types:
- Positive resonance effect in which the transfer of an electron is away from the atom or a substituent group.
- Negative Resonance effect in which the transfer of electrons is towards the atom or a substituent group.
The groups showing Resonance effect are –COOH, -CHO, -CN.
Fission of covalent bond:
Heterolytics cleavage: In the bond breaks in a way such that the shared pair of electron remains with one of the fragments.
CH3 –Br —————- +CH3 + Br-
Homolytic cleavage: In this the shared pair of electron goes to each of the bonded atom.
R –X ———– R. +X
Alkyl free radical.
Nucleophiles: A reagent which is responsible for bringing an electron pair is called nucleophile. It is also called as Nucleus seeking. Example –OH, -CN.
Electrophiles:It is a reagent that takes away electron pi. It is also called as electron seeking. Example C=O, CH3 – X
Inductive Effect: Inductive effect is the displacement of electron which in seen along the chain of the carbon atoms due to presence of an atom or group at the end of the chain.