Methods of purification
It is essential to purify an organic compound once it is extracted from a natural source or synthesised in the laboratory it. Various methods that are used for the purification of organic compounds are mostly based on the nature of the compound and the impurity present in it. The techniques used for purification are as follows:
-
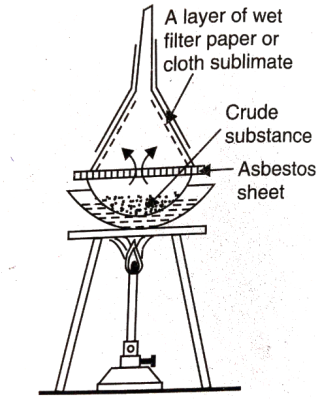
- Sublimation: Some solid substances change from solid to vapour state without passing through liquid state on heating. The purification technique based on the above principle is called sublimation. This method used to separate sublimable compounds from nonsublimable impurities.
- Crystallisation: It is one of the most popular methods used for purification. It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent. The impure compound is dissolved in a solvent in which it appreciably soluble at higher temperatureand sparingly soluble at room temperature. The solution is concentrated for saturating the solution. On cooling, pure compound will crystallize out and is removed by filtration.Impurities, which impart colour to the solution, are removed by adsorbing over activated charcoal. Repeated use of crystallisation is necessary for the purification of compounds containing impurities of comparable solubilities.
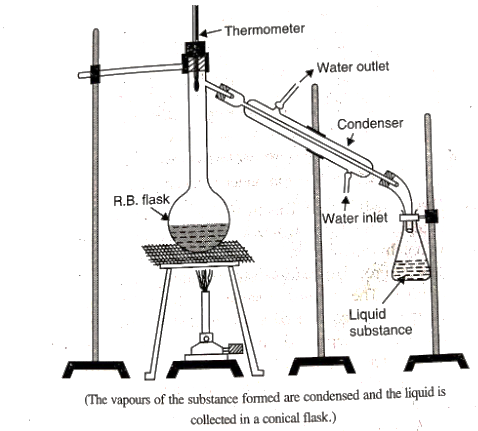
- Distillation: This important method is used to separate (i) volatile liquids from non-volatile impurities and (ii) the liquids having sufficient difference in their boiling points. Liquids having different boiling points vaporise at different temperatures. After cooling the vapours the liquids so formed are collected separately.
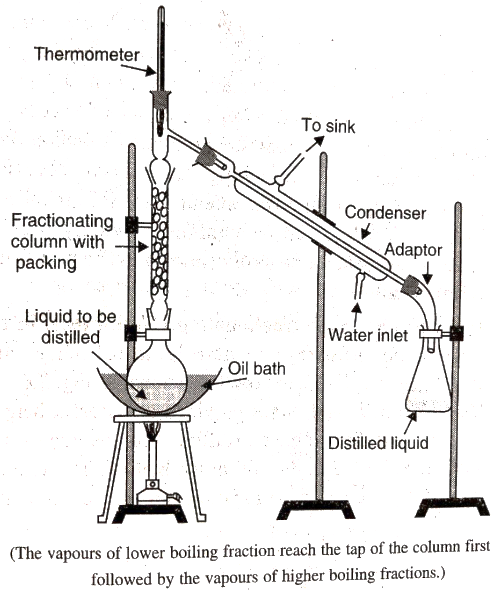
- Fractional distillation: If the difference in boiling points of two liquids is not much, simple distillation cannot be used to separate them as the vapours are formed at the same time. Fractional distillation is used in such cases. Here the vapours of a liquid mixture are passed through a fractionating column before condensation.Vapours of the liquid with higher boiling point condense before the vapours of the liquid with lower boiling point.
- Distillation under reduced pressure:This method is used to purify liquids having very high boiling points and those, which decompose at or below their boiling points. Such liquids are heated at a temperature lower than their normal boiling points by reducing the pressure on their surface.
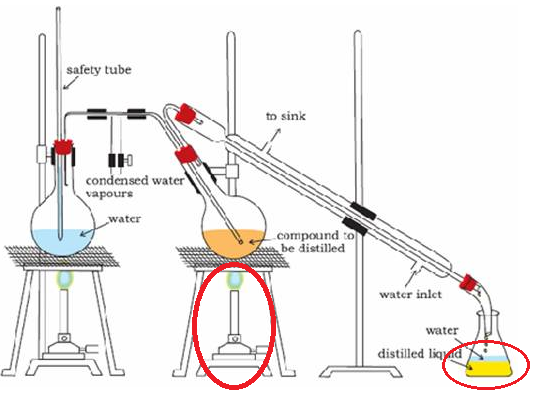
- Steam Distillation: This method is used to separate substances which are immiscible with water and are steam volatile.
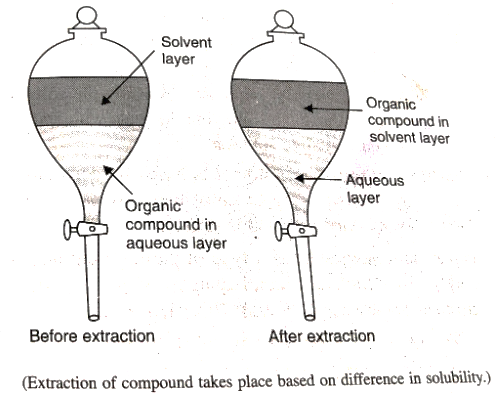
- Differential Extraction:An organic compound is separated from its aqueous solution by shakingit with an organic solvent in which it is more soluble than in water. The organic solvent is mixed with aqueous solution in a separating funnel and shaken and allowed to stand for some time. When water and organic solvent forms 2 different layers the lower layer is run out by opening the tap of funnel and organic layer is separated. This process is repeated several times.
- Chromatography: This technique is used to separate the mixtures into their components, purify the compounds and test the purity of compounds. It is classified as:
- Adsorption Chromatography
- Partition Chromatography
- Sublimation: Some solid substances change from solid to vapour state without passing through liquid state on heating. The purification technique based on the above principle is called sublimation. This method used to separate sublimable compounds from nonsublimable impurities.