There is an increasing tendency in the elements of group 14 to form the compounds with the +2 oxidation states. Particularly this phenomenon is prominent for lead and tin. Typically, the oxidation state adopted by the elements of this group is +4 as in SiCl4, SnO2, CCl4. However, methane is not the example of carbon having an oxidation state of +4. As electronegativity of carbon is more than the hydrogen so its oxidation state is -4. By moving down the group there are more examples of +2 oxidation state such as Pb2+, PbO, and SnCl2.
+4 oxidation state of tin is more stable than its +2 state but for lead and other heavy elements +2 oxidation state is more stable and due to this reason, the chemistry of lead is dominated. Carbon also has one common example of +2 oxidation state and that is carbon monoxide. It is a strong reducing agent as it is easily oxidized to the carbon dioxide which has a thermodynamically more stable state of oxidation that is +4. Many hot metal oxides are reduced to the elemental metals by carbon monoxide and this reaction is useful for many of the applications. Its most important use is the extraction of iron in the blast furnace.
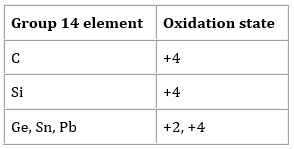
For tin and other elements lying below the tin +2 oxidation state is an increasingly common one and a variety of tin (II) and tin (IV) compounds is found. However, the oxidation state of tin (IV) is more stable. So, tin (II) compounds can be easily converted to the tin (IV) compounds. For example, a solution that contains the tin (II) ions and solvated tin (II) chlorides reduce the iodine to the iodide ions. In this process, the ions of tin (II) are oxidized to the ions of tin (IV). Also, tin (II) ions reduce the iron (III) ions to the iron (II) ions. Additionally, the ions of tin (II) are easily oxidized by the powerful oxidizing agents like potassium permanganate (acidified potassium manganate). In the organic chemistry, tin is used along with the concentrated hydrochloric acid for reducing nitrobenzene to aniline.
When it comes to the lead, the situation is reserved. The oxidation state of lead (II) is the more stable one. The tendency of lead (IV) compounds to react is also strong and as a result lead (II) compounds are formed. For example, lead chloride if decomposed at the room temperature, it gives chloride, chlorine gas and lead (II). When lead (IV) oxide is decomposed by heating then it gives oxygen and lead (II) oxide.
In general, there is nothing usual, for the stability of the +4oxidation state in this group. The outer electronic structure for each element in this group is ns2npx1npy1. Here n is the number of the period and it varies from 2 for the carbon and 6 for the lead. When the oxidation state is +4, all the electrons in the outer shell are involved in the bonding. At the bottom of this group, there is an increased tendency for s2 pair to uninvolved in the bonding. This is known as the inner pair effect and is dominant for the lead chemistry.