In the nomenclature of alkanes, the name of all alkanes ends with the suffix ‘ane’. Whether the alkanes are cyclic in nature or any chained alkanes with the absence of any double or triple bonds, the name of these alkanes always ends with the suffix and. This is also valid in whether the chain is branched or unbranched.
Structure of alkanes
The nomenclature of alkanes follows certain rules and methods to designate the names of the chain of alkanes. The alkanes with no sort of branching are simply named by the number of carbon atoms present in the chain. For the following number of carbon atoms in the chain, the naming is done as follows:
- CH4 Methane
- C2H6 Ethane
- C3H8 Propane
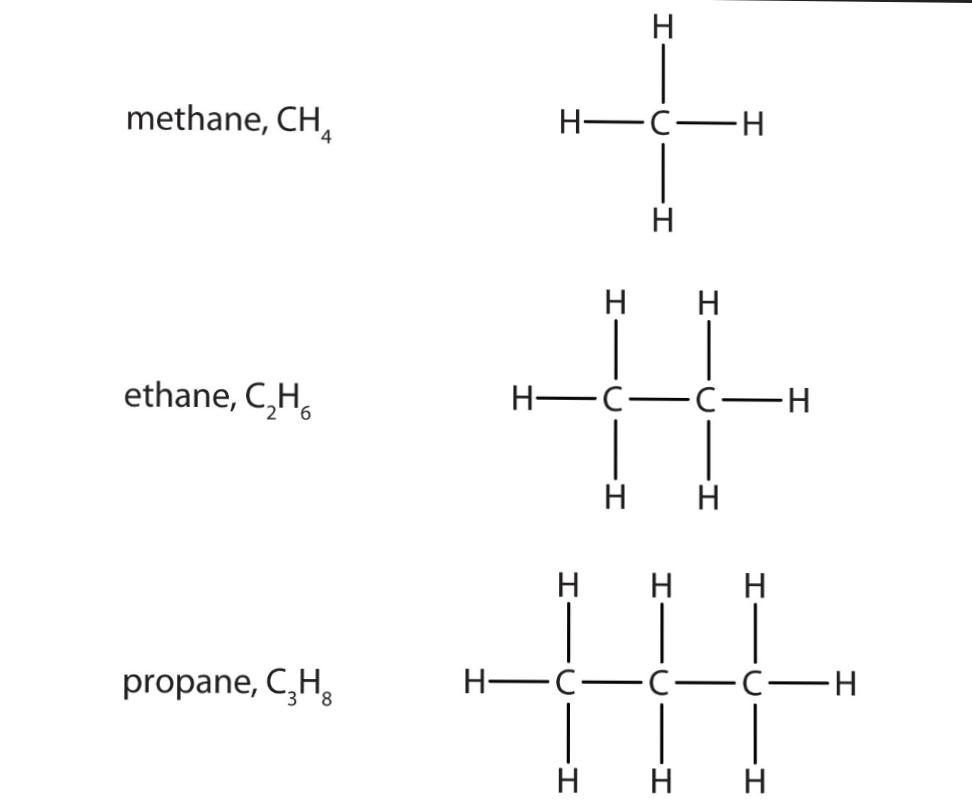
- C4H10 Butane
And so on.
The rule of equation followed here in the nomenclature of alkanes is Cn H 2n+2. Here the Cn portion represents the number of carbon on the structure of the compound and the H 2n+2 represents the number of hydrogen in the alkane structural compound. Similarly, we check for the propane compound.
Naming if complex compounds in the alkane nomenclature
This is a stepwise process hence firstly one should take into consideration the following process and follow it accordingly.
Step 1
Identify the longest chain in the alkane compound and designate it with the parent name. Such as if the longest chain includes 5 number of carbons then the parent name of the compound will be propane. For a different number of carbon atoms, there are different parent chain names. For this one should refer to the IUPAC parent name nomenclature of alkanes.
Step 2
Identify the substituent group attached to the parent chain. A substituent group is any other molecule attached in place of hydrogen in the parent chain by replacing the hydrogen atom.
Step 3
Do the numbering of the parent chain in such a manner that the substituent groups get the lowest possible rankings in the numbering process.
Step 4
Now name the entire compound. The parent name comes at the last position. Before the parent name cones the substituents groups names. The name of the substituent group is written in alphabetical order. Firstly the number of carbon on which the substituent group is designated us written. Then the corresponding substituent group’s name is written and then comes the parent chain name. If on a single carbon two similar substituent groups are placed then the word so is added in the name of the compound.
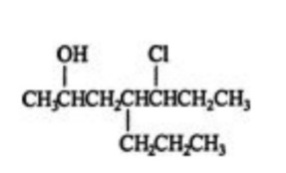
For example,
For the above compound, let’s do the alkane nomenclature, by following the above steps.
Let’s spot the longest chain in the compound. Comes to see that the longest carbon chain in the compound is of 7 carbons. Therefore the parent name of the compound will be heptane as 7 number of carbon atoms are present in the parent chain. Now let us number the chain in such a manner that the substituent atoms occur at lesser positions possible. Done with this let us name the compound. So the name of the alkane compound will be 5-chloro 2-hydroxy 4-propyl heptane.
Now to check the formula, substitute n equals to 1 then we get the final output as CH4 which is methane. Then substituting the value of n equals to 2 we get the output as C2H6 which is ethane.