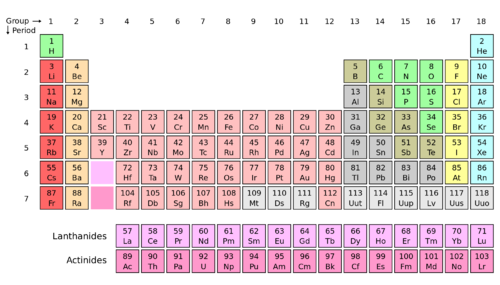
According to the Modern Periodic Table, there are total 118 elements present in it and are known to mankind. 24 elements out of 118 are prepared synthetically whereas the remaining elements have natural occurrence. A few of the elements were discovered formerly and some more elements are recently discovered by the team work lead by Glen Seaborg, working at the “Lawrence Berkeley Laboratory” in Berkeley, in California. A lot of the elements are assigned with their nomenclature/names plus the symbols, still these names and symbols are not universally used. So, a few of them are given two symbols or names.
Let’s say element having Z (atomic number)= 104 is asserted by the scientists of both American and Soviet union. Where Soviet named it Ku (Kurchatovium), the Americans nomenclatured it – Rf (Rutherfordium). In the same manner, another element having Z = 107 is assigned the name Ns (Neilsbohrium) along with Bh (Bohrium).
To erase these problems, IUPAC created the CNIC which is (Commission on Nomenclature of Inorganic Chemistry) to authorize a clear, nice, and better system of elements’ nomenclature that are having atomic number (Z) above 100, which are even called the “Superheavy elements.”
So, in 1997, after having a lot of discussion with all the scientists across the world, the IUPAC finally decided to give the official names for elements in the modern periodic table having atomic number starting from 104 to 110 and had also recommended a well-defined system for elements’s nomenclature.
The vital points to remember in this system of nomenclature for elements having atomic number greater than 100 are as follows:
1. Names can be written directly from their atomic numbers (Z) by scripting the numerical roots of the number required from no. 0 to 9 and after that you have to add a suffix – ‘ium’. The combination of Greek and Latin roots are used in order to avoid any duplicate thing found in the symbol. The roots starting from 0 to 9 are shown is the below-mentioned table.
Roots for IUPAC nomenclature of elements
| Digit | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Root | nil | un | bi | tri | quad | pent | hex | sept | oct | enn |
| Abbreviation | n | u | b | t | q | p | h | s | o | e |
2. In some of the cases, the names are reduced. For instance: bi ium can be represented as “bium”.
3. Element’s symbol can be specified by penning the roots of all numbers in the atomic no. of that particular element and by adding suffix “ium” to it.
4. The principle also says that the names must be short and of course should be related to elements’ atomic numbers.
5. The symbols must be directly derived from Z (atomic numbers) and should be related to the names as much as possible.
For instance:
| Z | Recommended name | Symbol |
| 101 | Unnilunium | Unu |
| 111 | Unununium | Uuu |
Or,
Here, are some of examples of the names created for elements with Z from 101 to 900.
| Atomic number | Name | Symbol |
| 101 | Unnilunium | Unu |
| 102 | Unnilbium | Unb |
| 103 | Unniltrium | Unt |
| 104 | Unnilquadium | Unq |
| 105 | Unnilpentium | Unp |
| 106 | Unnilhexium | Unh |
| 107 | Unnilseptium | Uns |
| 108 | Unniloctium | Uno |
| 109 | Unnilennium | Une |
| 110 | Ununnilium | Uun |
| 111 | Unununium | Uuu |
| 112 | Ununbium | Uub |
| 113 | Ununtrium | Uut |
| 114 | Ununquadium | Uuq |
| 115 | Ununpentium | Uup |
| 116 | Ununhexium | Uuh |
| 117 | Ununseptium | Uus |
| 118 | Ununoctium | Uuo |
| 119 | Ununennium | Uue |
| 120 | Unbinilium | Ubn |
| 121 | Unbiunium | Ubu |
| 130 | Untrinilium | Utn |
| 140 | Unquadnilium | Uqn |
| 150 | Unpentnilium | Upn |
| 160 | Unhexnilium | Uhn |
| 170 | Unseptnilium | Usn |
| 180 | Unoctnilium | Uon |
| 190 | Unennilium | Uen |
| 200 | Binilnilium | Bnn |
| 201 | Binilunium | Bnu |
| 202 | Binilbium | Bnb |
| 300 | Trinilnilium | Tnn |
| 400 | Quadnilnilium | Qnn |
| 500 | Pentnilnilium | Pnn |
| 900 | Ennilnilium | Enn |
Periodic Table