Every single environmental element has a specific role to play and complete several important reaction cycles. The destruction of any of these elements could create major imbalances.
Ozone Layer Depletion
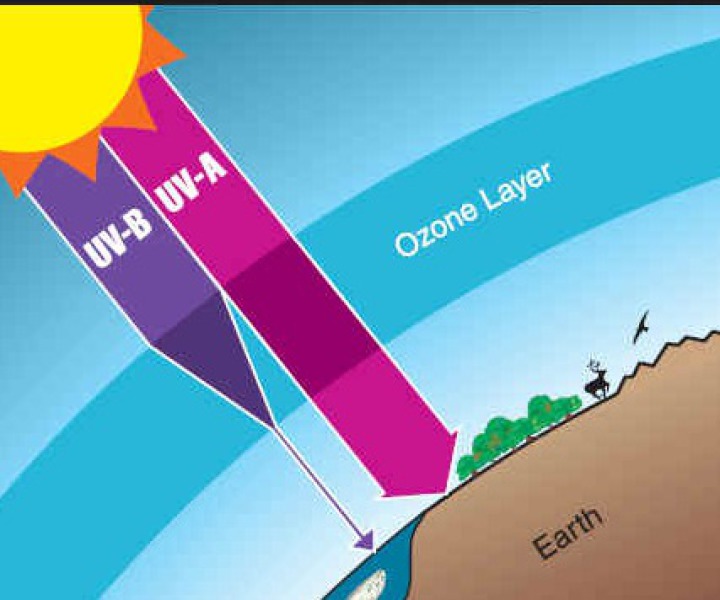
The ozone layer that prevails in the stratosphere is known to act like a protective shield for the earth against the harmful ultraviolet rays of the sun. The presence of Clorofluorocarbons (CFCs) is considered to be one of the major reasons for the depletion of ozone layer. These compounds tend to react under the presence of sunlight in order to form oxygen molecules. It’s been quite long since the scientists have discovered the ozone hole over the South Pole.
Once the ozone layer is entirely depleted, the ultraviolet rays of the sun would find their way directly to surface of earth. Being harmful, these rays tend to cause aging, skin cancer and other eye diseases like cataract and sunburn. Along with humans these rays are also harmful for animals. These ultraviolet rays cause the Phytoplankton to die which is responsible for the low productivity of fishes.
Causes and Effects of the Ozone Layer Depletion
Increased transpiration causes more loss of water via plants which turns the soil dry. Due to increased ultraviolet radiations, the protein cells in plants tend to undergo several harmful mutations. More ultraviolet radiations can also damage paints and fibers as a result of which they fade faster. Generally halocarbons and clorofluorocarbons are held responsible for the depletion of ozone layer.
However, the detailed study of this matter clarifies that this is caused more due to greenhouse gases. Greenhouse gases tend to absorb the heat that prevails in the atmosphere, consequently increasing temperature. This increase in the earth’s temperature causes ice caps to melt abnormally; increasing water level in oceans and seas. The coastal regions in that case are greatly prone to floods.
The Ozone Hole
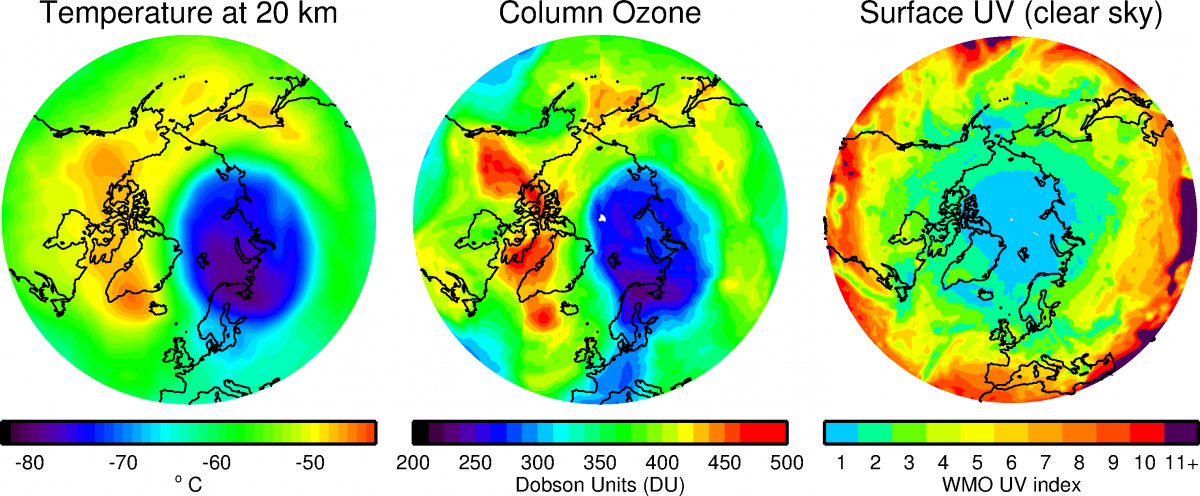
The depletion of ozone layer was first reported by scientists in the 1980s in Antarctica. This came to be known as the ozone hole. The depletion of ozone layer is caused due to considerable sets of climatic conditions.
During summers, the reaction of methane and nitrogen dioxide with chlorine monoxide and other chlorine atoms causes chlorine to shrink; subsequently preventing the depletion of ozone layer.
ClO (g) + NO2 (g) → ClONO2 (g)
Cl (g) + CH4 (g) → CH3 (g) + HCl (g)
During winters, special clouds tend to form over the Antarctic region. These create the situations that are favorable for the hydrolysis of chlorine nitrate which would eventually form hypolchlorus acid. Also, chlorine nitrate reacts with hydrogen chloride forming molecular chlorine.
ClONO2 (g) + H2O (g) → HOCl (g) + HNO3 (g)
ClONO2 (g) + HCl (g) → Cl2 (g) + HNO3 (g)
The sunrays enter Antarctica and break the clouds during springs. Photolysis for Cl2 and HOCl occurs, forming chlorine radicals which initiate depletion of the ozone layer.