Alkenes are organic compounds which contains double bonds in their chemical structure. They are also known as olefins interchangeably. They are class of unsaturated hydrocarbons that contains carbon and hydrogen atoms and have one or more carbon-carbon double bonds in its chemical structure. The unsaturation of Alkenes is credited of the presence of one or more double bonds in its structure. The general chemical structure of alkene is given as: RC=CR’. Acyclic alkenes are the subdivision of alkene containing only 1 double bond. However, it doesn’t consist of any other functional groups. And so it is also referred to asmono-enes.
The general formula of the homologous series of hydrocarbon is CnH2n.
Identification and naming of Alkenes becomes very important as they are huge in number and are used in our day to day lives. To identify them clearly, a systematic method of naming has been developed. It is called as the IUPAC (International Union of Pure and Applied Chemistry) system of nomenclature. In this systematic nomenclature, the names are dependent on the structure of the compound. Due to this the reader or listener can deduce the structure from its IUPAC name. Before the IUPAC system of nomenclature came into existence, organic compounds were assigned names based on their origin or certain properties.
A systematic name of an organic compound is generally derived by the identification of the parent hydrocarbon and the functional groups attached to it.
The parent name can be modified to obtain the actual name, by further using prefixes and suffixes
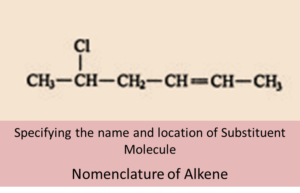
IUPAC Nomenclature of Alkenes:
For nomenclature of alkenes in IUPAC system, following steps can be followed:
- The longest chain of carbon atoms which contain the double bond is identified and selected.
- The suffix ‘ene’ replaces ‘ane of alkanes.
- The names of the alkyl groups attached to a branch are then prefixed to the name of the parent alkene. Its position is indicated by numbers.
- Numbering of the carbon starts from the side nearest to the double bond in the chain.
- The carbon atom of the branch that is attached to the root alkene is numbered 1.
It must not be forgotten that first member of alkene series is: CH2. It is obtained by replacing n by 1 in CnH2n and is known as methene but has a very short life. As mentioned the first stable member of alkene series is C2 H4 known as ethylene (common). Its IUPAC name is ethene. IUPAC names of some of the members of alkenes are as follows:
Structure IUPAC name
Que) Find out the IUPAC names of the following compounds:
Solution
- 2,8-Dimethyl-3, 6-decadiene;
- 1,3,5,7 Octatetraene;
- 2-n-Propylpent-1-ene;
- 4-Ethyl-2,6-dimethyl-dec-4-ene;