Chemical Reactions including free radical mechanism of halogenation.
Alkyl halide (halo alkane) is formed by the replacement of hydrogen atom in a hydrocarbon, by halogen atom. It contain halogen atom attached to the carbon atom of an alkyl group which is sp3 hybridised.
Alkanes are generally non-reactive when it comes to acids, bases, oxidising and reducing agents. However, they undergo the following reactions under certain conditions.
Substitution reactions
In substitution reaction one or more hydrogen atoms of alkanes are replaced by halogens, nitro group and sulphonic acid group. Nitration and sulphonation reactions are not seen for lower alkanes. The following reactions in which hydrogen atoms of alkanes are substituted are known as substitution reactions.
Halogenation:
Halogenation takes place either or in the presence of diffused sunlight or ultraviolet light or at higher temperature (573-773 K). Chlorination of methane is given below as an example:
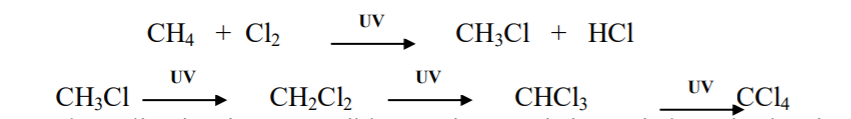
It is observed that the rate of reaction of alkanes with halogens is in the order of F2 > Cl2 > Br2 > I2. Rate of replacement of hydrogen of alkanes is : 3° > 2° > 1°. Fluorination is too violent to be controlled. Iodination is very slow and a reversible reaction. It is mainly carried out in the presence of oxidizing agents like HIO3 or HNO3.
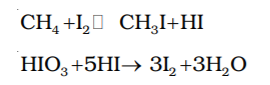
Halogenation is supposed to take place via free radical chain mechanism involving three steps namely initiation, propagation and termination as given below:
Mechanism
- Initiation: The reaction is initiated by homolysis of chlorine molecule in the presence of light or heat. The Chlorine–Chlorine bond is weaker than the Carbon-Carbon and Carbon–Hydrogen bond and hence, is easiest to break.
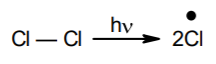
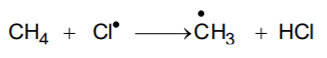
- Propagation: Methane molecule is attacked by chlorine free radical and takes the reaction in the forward direction by breaking the C-H bond to generate methyl free radical with the formation of H-C.
 Thus the methyl radical obtained attacks the second molecule of chlorine and forms CH3 – Cl (methyl chloride) liberating another chlorine free radical by homolysis of chlorine molecule.
Thus the methyl radical obtained attacks the second molecule of chlorine and forms CH3 – Cl (methyl chloride) liberating another chlorine free radical by homolysis of chlorine molecule.
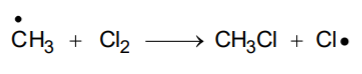
The chlorine and methyl free radicals are generated. Repeat the above step and thereby setup a chain of reactions. Propagation steps leads to the formation of principal element, but other propagation steps are also possible. Two such steps are given below. It explains how to form more highly halogenated products.
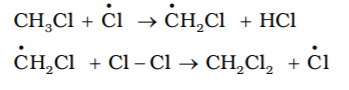
- Termination: The reaction will stop after some time. This is due to consumption of reactants and / or due to the following side reactions:The possible chain terminating steps are:
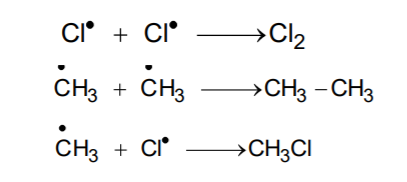
Though in (c), CH3 – Cl, the one of the products is formed but free radicals are consumed and the chain is terminated. The above mechanism helps us to understand the reason for the formation of ethane as a by-product during chlorination of methane.
Nitration:
The reaction takes place by free radicals mechanism at high temperature around 450o C. At high temperature Carbon – Carbon bond is also broken so that we obtain the mixture of nitroalkanes.
Sulphonation Reaction:
It is basically the replacement of hydrogen atom of alkane by –SO3H group.
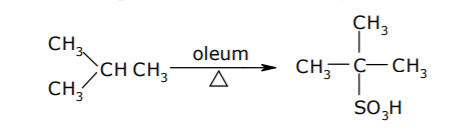
The reaction occurs as: