Organic chemistry – Electromeric effect
An Electromeric effect is a temporary effect that takes place between two atoms joined by multiple bonds for means a double or triple bond. This occurs at the requirements of the attacking reagent and involves an instantaneous transfer of a shared pair of electrons of the multiple bonds to one of the linked atoms. However, when attacking reagent is removed, the molecule acquires its original electronic condition.
As an example consider the carbonyl carbon –C=O, present in aldehydes and ketones. When a negatively charged reagent seeking positive site, say approaches the molecule, it causes an instantaneous shift of electron pair of carbonyl carbon group to the oxygen more electronegative than carbon. As a result, the carbon becomes deprived of its share in this transferred pair of electrons and thus acquires a positive charge while oxygen which has taken complete control of the electron pair becomes negatively charged. Therefore, in the presence of attacking reagent, one bond is lost. Then the negatively charged attacking reagent links to the carbon having a positive charge.
Thus, this movement of electrons from one atom to another in a multiple bonds at the demand of attacking reagent is called electromeric effect and is denoted by E effect. The electromeric shift of electrons occurs only at the moment of the reaction.
Electromeric effect is also differentiated into two types:
- Positive electromeric effect or +E effect
- Negative electromeric effect or -E effect
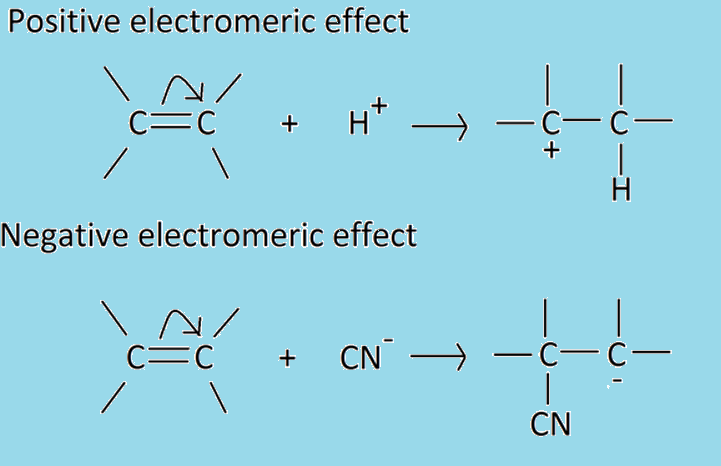
Positive electromeric effect:
When the electrons of π-bond are transferred to the atom to which attacking reagent finally gets attached, the effect is called positive electromeric effect or +E effect. For example, the addition of acids to alkenes.
Negative electromeric effect:
When the electrons of the π-bond are transferred to the atom other than the one which the reagent finally gets attached to, the effect is called negative electromeric effect or –E effect. For example, the addition of cyanide ion to the carbonyl group. When a negatively charged reagent approaches a molecule seeking partially positive carbon, it causes an instantaneous shift of an electron pair of the C=O group towards the more electronegative oxygen atom. Hence, carbon becomes deprived of its share in this transferred pair of electrons and acquires a positive charge. At the same time, oxygen takes complete control of the electron pair and becomes negatively charged. As a result, in the presence of attacking reagent, one bond is lost and this negatively charged attacking reagent links to the carbon having a positive charge. It is temporary in nature because the molecule acquires its original electronic condition upon removal of attacking reagent.
Thus, it may be concluded that the electromeric effect is said to be the +E effect when the displacement of electrons is towards the atom or group and –E effect when it is away from the atom or group on which attacking reagent is added.
Now let us go through characteristics of the electromeric effect from our discussion. Here are a few of them:
- It is a temporary effect that involves a complete transfer of π-electrons to one of the bonded atoms.
- It operates in unsaturated compounds which contain at least one multiple bonds which may be polar or non-polar.
- It operates only in the presence of an attacking reagent.
- It involves a complete transfer of electrons from the reagent to the substrate and vice versa. As a result of this effect, ions are formed.