Organic chemistry – Resonance and Hyperconjugation
Resonance or mesomeric effect:
There are many molecules whose behavior cannot be explained by a single Lewis structure. To explain their behavior two or more than two structures may be proposed and the actual molecule is said to be a resonance hybrid of these structures. This phenomenon is called resonance. Thus if a molecule can be assigned two or more structures, none of which capable of describing all the known properties of the compound, then the actual structure is intermediate or resonance hybrid of these structures. This phenomenon is called resonance. The various structures written are called resonating structures.
The phenomenon of resonance can be explained with the help of the molecule. It has been represented by the structural formula:
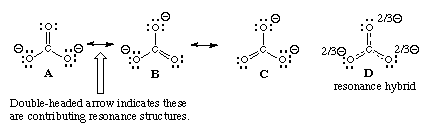
Though this satisfies the conventional valency requirements, yet it cannot explain some experimental facts. The actual carbon-oxygen bond in has been found to be 115pm whereas the normal carbon-oxygen double bond is 122pm and that for the triple bond is 110pm. This means that carbon-oxygen bond in carbon dioxide is intermediate between a double and a triple bond.
Let us consider another example of benzene. The resonating structures are shown below:
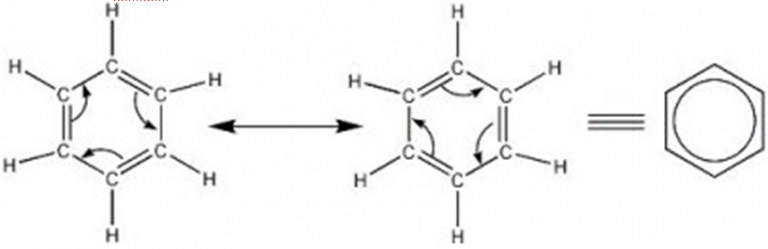
It may be noted that the energy of the actual structure of the molecule means a resonance hybrid is lower than that of any of the resonating structures. The difference in energy between the actual structure and the most stable of the resonating structures is called resonance energy or resonance stabilization energy. The different resonance structures contribute to the actual structure in proportion to their stability.
There are two types of resonance effect:
- Positive resonance effect (+R effect): If a substituent has the tendency to donate electrons to double bond or conjugated system, the effect is called positive resonance effect. For example, groups such as
etc show the +R effect.
- Negative resonance effect (-R effect): If a substituent has the tendency to withdraw electrons from a double bond or a conjugated system towards itself, the effect is called negative resonance effect or –R effect. For example, groups such as
etc, show the –R effect.
Hyperconjugation:
When the H-C bond is attached to an unsaturated system such as a double bond or a benzene ring, the sigma electrons of the H-C bond interact or enter into partial conjugation with the unsaturated system. The interaction between the electrons of π systems and the adjacent σ bonds of the substituent groups in organic compounds is called hyperconjugation. The two scientists named Baker and Nathan first developed the concept of hyperconjugation, hence it is also known as Baker-Nathan effect.
According to this concept, if an alkyl group carrying at least one hydrogen atom is attached to an unsaturated carbon atom, it releases electrons of carbon-hydrogen single bond towards the multiple bonds. Let us take one example of hyperconjugation. The hyperconjugation in propene has been shown below:
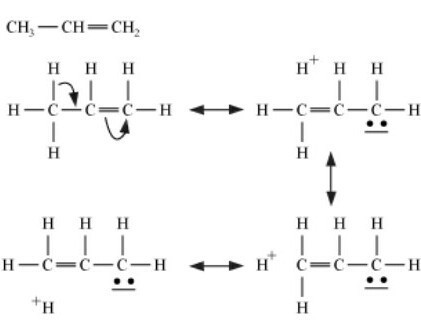
The various hyperconjugation forms of propene are called contributing structures or hyperconjugating structures. The hyperconjugation effect is almost similar to the resonance effect. As we can observe there is no bond between the α-carbon atom and one of the hydrogen atoms, the hyperconjugation is also called no-bond resonance. However, it may be noted that although a free proton has been shown in the above structures, it is still bound quite firmly to the π-cloud and hence it is not free to move. It is clear from the above structures that hyperconjugation occurs through the H-atoms present on the carbon atom next to the double bond is α-hydrogen atoms. Therefore, more number of α-hydrogen atoms, more are the number of hyperconjugative structures. Thus, the hyperconjugation effect is expected to be more due to the α-carbon atom.