Covalent Network Solids
Covalent solids are composed by a series or chain or network of atoms or molecules which together held by covalent bonds. An ideal individual crystal of covalent solid is hence one single huge molecule. For instance, the diamond structure is shown in part (a) of Fig. 1, which contains sp3 hybridization of (C) carbon atoms, each of the atom is covalently bonded to 4 other carbon atoms in Tetrahedral array to build a very large network.
The atoms of carbon form 6-membered rings
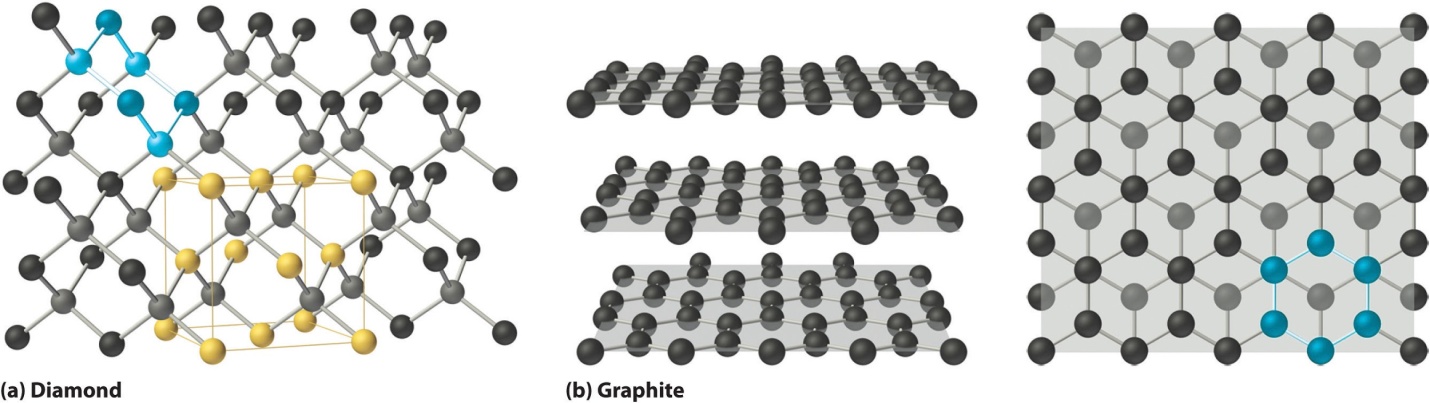
As all the bonds of the diamond structure are strong on an equal level, the covalent solids are often very rigid and it’s a pretty tough task to melt them. Diamond is known to be the hardest substance present naturally and it melts at about 355-degree Celsius.
Carbon: An eg. of the Covalent Network Solid
In network solid types, traditional chemical bonds together hold chemical subunits. The bonding or relationship between the chemical subunits, nevertheless, is similar within the subunits, which results in an unending network of chemical bonds.
A common example of network solids is diamond (pure form of carbon). Carbon is having its existence as a pure element under room temperature conditions in 3 different forms: diamond, graphite (most stable form), and fullerene.
Metallic Solids
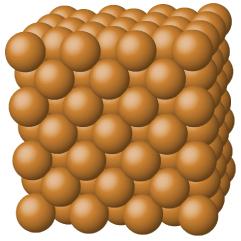
Fig : Metallic solid figure of Copper
Metallic solids including aluminum, copper, and iron, or alloys are made up of metal atoms of metal. Figure 2. Metal crystals’ structure is oftentimes illustrated as the even distribution of atomic nuclei inside the “sea” of delocalized electrons.
Within such metallic solids, atoms are put together by an exclusive force called, metallic bonding which results in several useful and diverse bulk properties. All display high electrical and thermal conductivity, malleability, and metallic lusture.
Many of them very hard and strong enough. Due to their malleability, they don’t break into small pieces and hence, make beneficial construction materials.
Metals are identified by their capability to reflect light, which is called lusture. Also, characterized by their extreme heat capacity, high thermal and electrical conductivity, and their ductility and malleability.
Each of the lattice point is occupied by the atom of identical metal in a genuine metallic element. Metal crystals have more packing efficiency, therefore the metallic solids are more compact, dense, where each atom is having considerable 12 closest neighbors.
The important thing to notice is that, Metallic solids having valence electrons are delocalized, which provides a robust cohesive force which together holds atoms.