A regular 3-D arrangement of ions or atoms or molecules in the crystal is generally described in the context of a unit cell and the space lattice. To understand these two terms, let’s take 2-D patterns
Crystalline solids’ geometric arrangement belonging to constituent particles in the form of a point in space is called crystal lattice.
Characteristics of crystal lattice:
- Each of the constituent particles in a crystal lattice is depicted by a single point
- Those single points are called the lattice site or the lattice point
- In a crystal lattice, the lattice points are together joined by straight lines
- Crystal lattice’s geometry is formed by joining lattice points with the help of straight lines
When you think of three structures individually with a extensive number of repetitions in 2 directions appropriate to parallel-sided figure which is instantly shown below every pattern.
2-D crystal structures
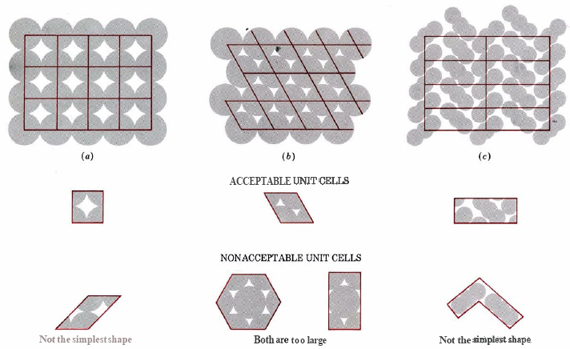
Fig: 2-D lattices’ unit cell.
The unit cell is this parallel-sided picture. It clearly depicts the smallest, simplest shape from which an entire structure can be composed. The lines which are joining every point of space lattice are represented in color (specifically red color). Without having a little bit of experience, it’s pretty easy to choose the incorrect unit cell for a already given structure.
Unit Cell: The littlest or the smallest part of the crystal lattice is referred to as the Unit Cell. By the repetition process in different directions, the unit cell produces whole lattice.
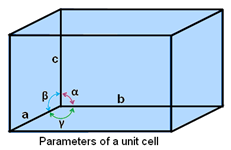
Parameters of a unit cell:
- One unit cell is described by 6 parameters. These parameters are defined as the three edges, namely a, b, and c; and the angles between them are named as α, β, and γ.
- Alongside the edges, dimensions of the unit cell are characterized by a, b, and c.
- Unit cell’s edges are having or not having the possibility of being mutually perpendicular
- The angle created between edges b and c, are given by “α”, whereas, between c and a by “β”, “γ” is the representation of angle between edges a and b.
Types of Unit Cell:
Basically, 2 unit cells types are present called, the Centred and Primitive Unit Cells
Primitive Unit Cells: When unit cells’ particles find themselves on the corners only, then it is referred to as the primitive unit cells.
Centred Unit Cells: When these unit cell’s particles are having their presence at different positions, likewise to those present at unit cell’s corner, then it’s referred to as the Centred Unit Cell.
There are three types of Centred Unit Cell
- Body Centred Unit Cells
- Face-Centred Unit Cells
- End-Centred Unit Cell
In general 7 types of unit cells are formed. They take shape in the form of Cubic, Hexagonal, Tetragonal, Monoclinic, Orthorhombic, Trigonal or Rhombohedral or Triclinic.
A common formula which is presented for N counting, from the arguments, and the ‘n’ number of ions or atoms present in a unit cell are given by:
N=Nbody + Nface2 + Nedge4 + Ncorner8