Packing in solids can illustrate the crystalline solid structures. When comes the crystal structure, the centers of ions, molecules, atoms are located on the lattice points.
Atoms are presumed to be spherical to describe the structures and bonding of metallic crystals. The sphere’s arrangement in closed-packed structures are packed densely so as to undertake the greatest space amount as much it’s possible.
The Principle of Packing in Solids:
- Particle whether ions, atoms, or molecules in a crystal, is filled with lattice points in a crystal lattice.
- To attain the maximum closed-packed structure, the constituent particles try their best to be packed together very closely.
- Minimum space which is empty is left when there’s maximum closed-packed structure.
- Because of this arrangement, the maximum possible crystal density is attained
- Close packing relates to crystal’s stability.
Closed Packing in One Dimension:

- Only one method is there to arrange spheres in a 1-D close-packed structure, which is to organize them in a single row or line and having contact with each other.
- Regarding this arrangement, each of the spheres makes contact with its two neighbors, present on the left and right-hand side. The closest neighbors’ number of the particle is known as its Coordinate Number. Therefore, in a 1-D close-packed arrangement, the coordinate number, also called the “Ligancy” is “2”.
Closed Packing in Two Dimension:
- A 2-D closed-pack structure can be created by arranging or piling up the closed-packed spheres rows. It can be achieved in two different methods.
Types:
Close Packed Structure
In close-packed structure, matters are in solid state due to close packing of respective their constituent particles. Two types of close packing are found in solid substances. These are namely Cubic Close Packed (ccp) and Hexagonal Close Packed (hcp) lattice.
Cubic Close packed (ccp):
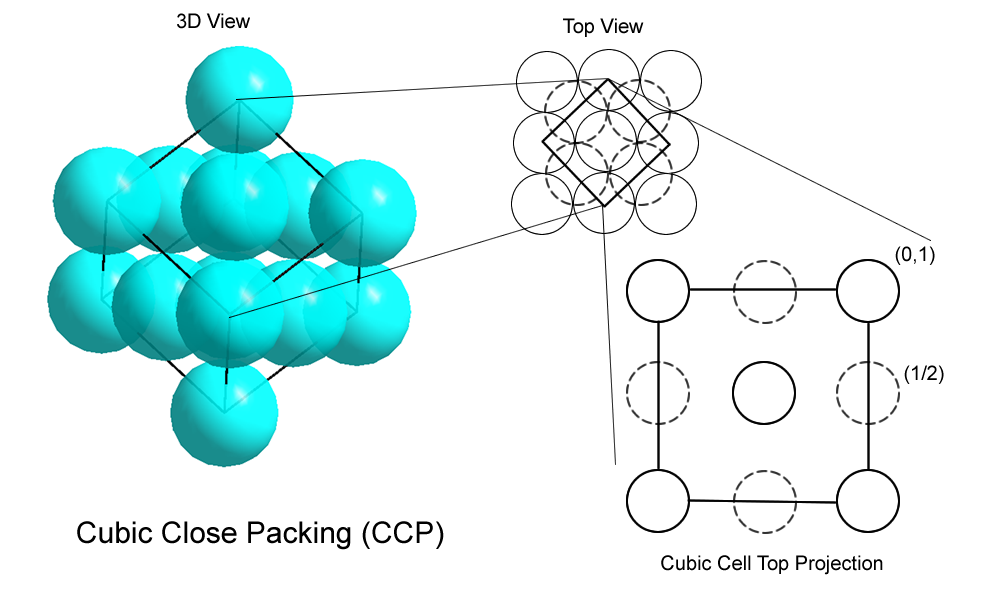
This type of packing have molecules’ sphere, placed adjacent with one another, so that sphere’s each row in a specific dimension is a result of repetition of the past row.
In this type of arrangement, each sphere which is in touch with 4 of its adjacent spheres. Hence, the 2-D “coordination number” is 4. Also, when 4 of the neighboring spheres are joined together as per the diagram above, a square forms and hence the packing is aslo called “square close packing” in 2-D.
This arrangement is also called as AAA type of arrangement.
Hexagonal Close packed (hcp):
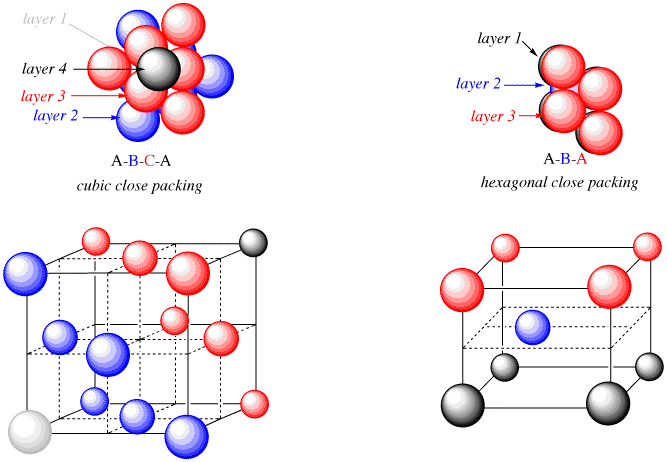
This packing type in a specific dimension relates to the molecules’s sphere of a particular row in a specific dimension are in a state/position where they are fitted into depressions in between previous row’s adjacent spheres. So, this arrangement type is known as “ABAB type arrangement”.
This packed lattice type is seen in various metals like Zinc, Magnesium, etc.
Coordination number:
Coordination number is defined as adjacent particles’ number of atoms which is known as the Co-ordination Number