Chemical Kinetics: Factors affecting the rate of a reaction
Catalyst
The rate of a reaction can be dramatically increased by supplying heat energy and/or increasing the concentration of reactants. However, heat gain is not always the best or the most practical thing to do. For example, suppose that you want to increase the rate of a reaction such as the decomposition of glucose in a living cell. Increasing the temperature and/or the concentration of reactants is not always a viable alternative as it might harm or kill the cell. Many chemical reactions in living organisms would not occur quickly enough to sustain life at normal living temperatures without enzymes. An enzyme is a type of catalyst. A catalyst is a substance that alters the rate of a chemical reaction without itself being used. It provides a good “environment” for a reaction to occur, thereby increasing the reaction rate without being consumed by the reaction.
A catalyst provides an alternative reaction pathway to the reaction in order to lower its activation energy and increases the reaction rate. Also, a catalyst does not affect the reaction enthalpy or the equilibrium position and the reaction yield remains the same at equilibrium.
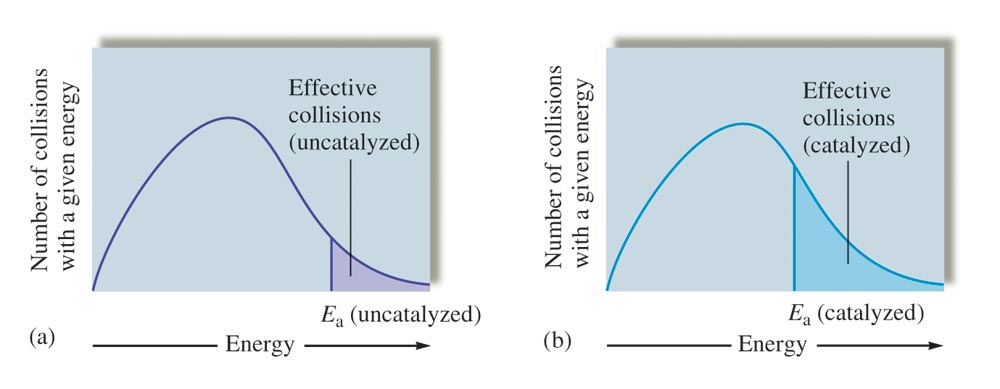
Fig 1: Effect of catalyst on the number of the reaction producing collisions.
The energy diagrams below show how the activation energy of the catalyzed reaction is lower and therefore the reaction produces the products at a faster rate than the uncatalyzed reaction does. Accordingly, Ea(catalyzed reaction )<Ea (uncatalyzed reaction) which indicates that more collisions have enough energy to initiate the reaction in the presence of a catalyst.
Although catalysts play an important role in a chemical reaction, they do not yield more product than the uncatalyzed reaction. The catalyst participates in at least one step of the reaction and then regenerate in a later step to avoid its consumption. Its presence increases the rate of reaction by either increasing the frequency factor, A (from the Arrhenius equation) or lowering the activation energy, Ea.
Homogeneous catalysts react with the reactants in the same phase. For instance, the formation of SO3 from SO2 and O2 is an exothermic reaction with a very high Ea. AT low temperatures, the reaction rate is very low while the increase in temperature reduces the yield of products. In this case, the addition of nitric oxide leads to the formation of transition-state complexes that have lower activation energy. These complex ions make the reaction go faster at a moderate temperature.
Most reactions involving gases use inert metals or metal oxides as heterogeneous catalysts. These solid heterogeneous catalysts provide surface areas for optimum molecular interactions and facilitate the breaking and formation of bonds. Such surface catalysis is thought to occur by chemical adsorption of the reactants onto the surface of the catalyst. Adsorption is the attraction of molecules to a surface. For examples, Ni, Pd and Pt are often used in the hydrogenation of vegetable oil for making Crisco oil and margarine.