Aromaticity is a term used in the organic chemistry for describing the cyclic and the planar molecules which have the resonance bonds and exhibits the more stability than the geometric or connective arrangements in the same kind of the atoms. Aromaticity is the property of conjugated cycloalkenes that enhances the stabilization of the molecules. The aromatic molecules are much stable and cannot be broken easily. Aromatic molecules also react with the other type of substances.
Usually, people encounter the aromaticity in daily life. This phenomenon is extremely important for the normal functioning of human bodies. These aromatic compounds are also essential for the industry. Every year in the form of chemicals and polymers such as polyester and nylon these compounds are produced worldwide with an estimated amount of around 35 million tons.
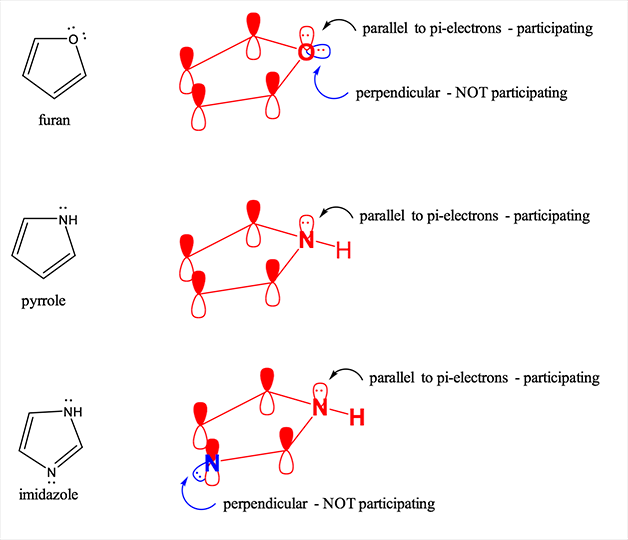
Rules of the Aromaticity
The compounds showing aromaticity are less stable, and they have numerous synthetic and chemical uses. The aromatic compounds have some special characteristics and which are generally termed as the aromaticity rules and are given below.
- The aromatic compounds always have cyclic structures.
- Within the structure, each element of the ring must have p orbital ring and it should be in the perpendicular form to the ring. Due to this reason the planar molecule is formed.
- According to the Huckel’s rule all of the aromatic compounds should have 4n+2 number of the pi electrons.
- The organic compound should be flat.
Importance of the Aromaticity
Aromaticity has a significant role in all the living structures and the field of biochemistry. There are four kinds of the aromatic amino acids that are phenylalanine, tyrosine, histidine, and tryptophan, which serves as twenty basic types of building blocks for the formation of proteins and proteins are building blocks of the life are also aromatic compounds and all of the nucleotides that makes the RNA and DNA sequences are aromatic. In short, aromaticity is vital for many aspects of life.
The green pigment in the plants known as chlorophyll has the aromatic system. Aromatic compounds have significant importance in the industry. The most commonly used aromatic hydrocarbons for commercial interests are ortho-xylene, para-xylene, benzene, and toluene. These aromatic hydrocarbons are produced from the complex mixtures, obtained from the process of distillation, and refining, and are used for the production of different kinds of chemicals such as aniline, styrene, polyester, nylon, and phenol.
Benzene is also an aromatic compound and it has many distinctive features among which its aromaticity is a major contributor for its un-reactivity. The molecule of benzene is planar due to the aromaticity. Another important aspect is that there are no distinctive single or double bonds in the benzene molecule. Due to the delocalization the ring counts each as one and a half bonds between the carbons and it makes sense as experimentally, the actual bonding length is between the single and the double bond and finally in its structure there are six electrons of p orbitals which are responsible to make the stabilizing cloud of electrons below and above the aromatic ring.