Alkenes are more reactive than the alkanes as in their pi bonds the electron density is available. These compounds can participate in the variety of the addition reactions and they can also be used for making the polymers.
Addition Reactions
Alkenes participate in the many addition reactions. The unsaturated hydrocarbons can help in the number of various addition reactions across their triple or double bonds. These addition reactions include the halogenation, hydrogenation, and the hydrohalogenation.
Cycloaddition
Alkenes undergo various cycloaddition reactions. Here, the most notable fact is that they undergo the Diels Alder reaction with the 1,3 dienes and give the cyclohexenes. The process of cycloaddition of the terminal alkenes convert them to the cyclobutanes. The cycloaddition of the diene alkene converts them to the vinyl cyclobutanes products.
Oxidation
At low temperatures, the alkenes can be oxidized and give the glycols. The glycol will be further oxidized at the higher temperatures and they will yield a carboxylic acid and the ketone.
Hydrogenation
The reaction of alkenes with the hydrogen produces the alkane. For example, when propene reacts with hydrogen it gives propane.
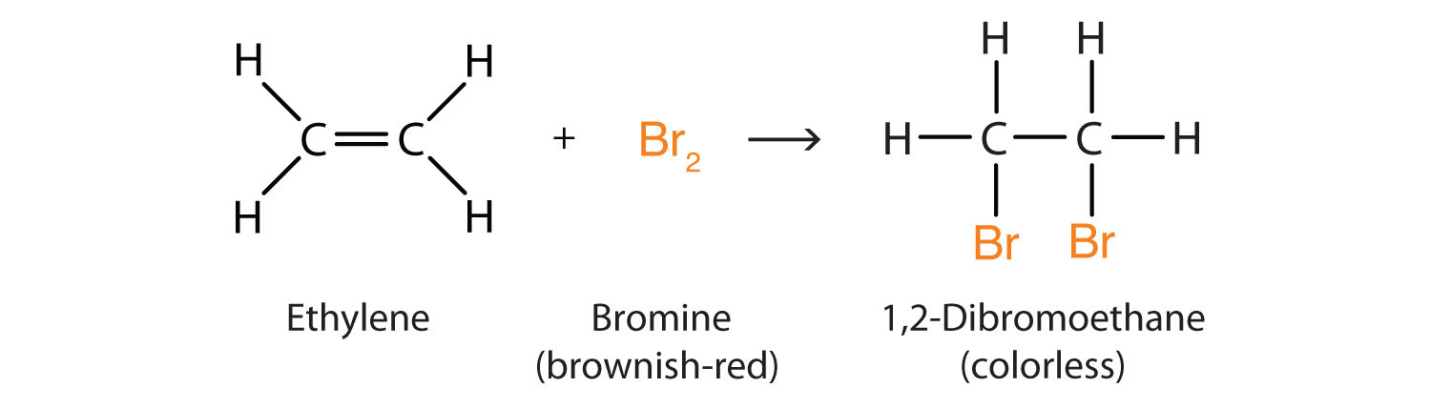
Halogenation
The alkenes can be halogenated by the addition of halogen across the double or triple bond in the same fashion as that of hydrogenation. The result of halogenation of the alkene is an alkane product that is di-halogenated. The reaction of alkenes with the halogens cause the removal of the functional group of the alkene. For example, the reaction of ethene with the bromine produces the dibromoethane. Here di refers to the presence of two bromine atoms in the compound and ane shows that carbon double bonds are no longer present there. Additionally, its functional group is the same as that of alkane.
Hydrohalogenation
Alkenes react with the hydrogen halides and like HBr and HCl. The reaction of hydrohalogenation produces the corresponding alkyl dihalides or the vinyl halides but it is strongly dependent on the number of the added HX equivalents. If the symmetry of the alkene is asymmetric the reaction will follow the rule of the Makrovinkov i.e. the addition of halides to the carbon. Markovnikov’s rule states that if the hydrogen halide is added to the alkene it will lead to the formation of the product in which hydrogen is attached to the carbon with the few alkyl substituents whereas halide group is attached to the carbon with the more alkyl substituents.
Hydration
The hydration of the alkenes by the oxymercuration process yields alcohol. Generally, this reaction takes place by the treatment of the alkenes with the strong acid which works here as a catalyst.
Combustion of Alkenes
The oxygen in the air is not enough for the complete combustion of alkenes, so, its combustion in the air will only produce the water, and carbon dioxide. Just like the other combustion reactions, this reaction is also exothermic and gives out the energy. Their burning in the air produces the yellow smoky flames. The amount of the products formed strongly depends on the amount of oxygen, so, there may be reactions with no production of the carbon.